Decomposition Of Lead Iv Oxide Balanced Equation . For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. The balanced chemical equation for the decomposition of lead (iv) oxide is pbo₂ (s) → pb (s) + o₂ (g), which is correctly balanced with one lead atom and two oxygen atoms on each side. The balanced equation will appear above. The balanced chemical equation representing the decomposition of lead (iv) oxide is option a: This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. Pbo2 = pb + o2. The balanced chemical equation for the decomposition of lead iv oxide to yield lead ii oxide and a colorless gas is: Consider as an example the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. See examples of decomposition reactions, such as.
from www.numerade.com
The balanced chemical equation representing the decomposition of lead (iv) oxide is option a: This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. The balanced chemical equation for the decomposition of lead iv oxide to yield lead ii oxide and a colorless gas is: A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear above. See examples of decomposition reactions, such as. Consider as an example the. Pbo2 = pb + o2. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge.
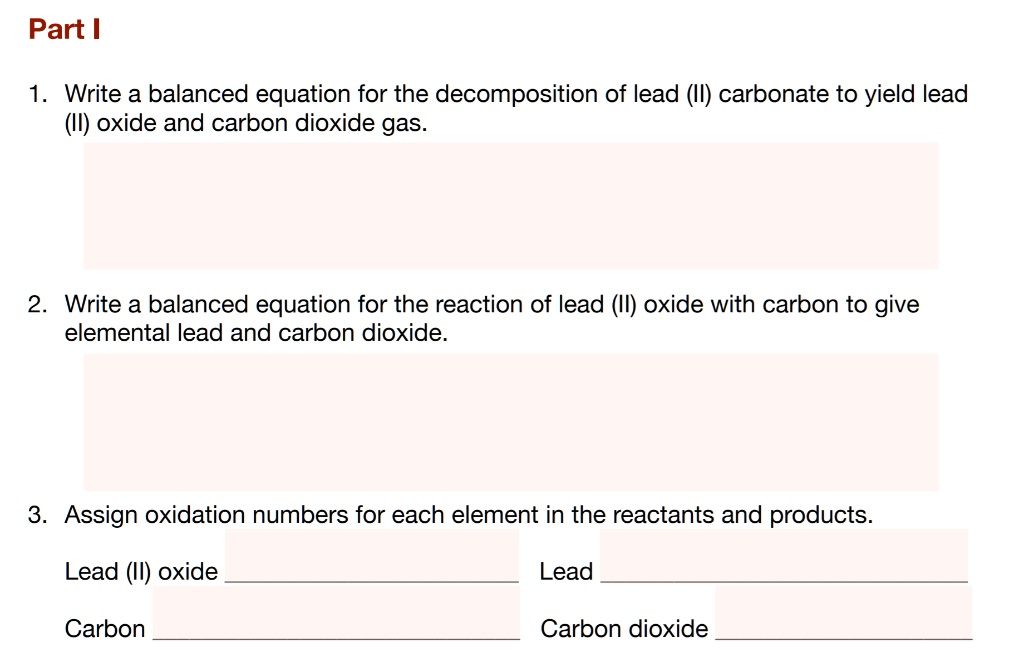
SOLVED Part Write a balanced equation for the of lead
Decomposition Of Lead Iv Oxide Balanced Equation Consider as an example the. This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. Pbo2 = pb + o2. Consider as an example the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced chemical equation representing the decomposition of lead (iv) oxide is option a: The balanced chemical equation for the decomposition of lead iv oxide to yield lead ii oxide and a colorless gas is: The balanced equation will appear above. The balanced chemical equation for the decomposition of lead (iv) oxide is pbo₂ (s) → pb (s) + o₂ (g), which is correctly balanced with one lead atom and two oxygen atoms on each side. See examples of decomposition reactions, such as. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge.
From www.numerade.com
SOLVED 0) 3 Write balanced chemical equation for each of the following Decomposition Of Lead Iv Oxide Balanced Equation See examples of decomposition reactions, such as. This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. Consider as an example the. The balanced equation will appear above. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Pbo2 = pb + o2. For each p b4+. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.numerade.com
SOLVED Write a balanced equation for each of the following Decomposition Of Lead Iv Oxide Balanced Equation The balanced chemical equation for the decomposition of lead (iv) oxide is pbo₂ (s) → pb (s) + o₂ (g), which is correctly balanced with one lead atom and two oxygen atoms on each side. The balanced chemical equation for the decomposition of lead iv oxide to yield lead ii oxide and a colorless gas is: Pbo2 = pb +. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.numerade.com
SOLVED Write a balanced chemical equation based on the following Decomposition Of Lead Iv Oxide Balanced Equation See examples of decomposition reactions, such as. The balanced chemical equation for the decomposition of lead (iv) oxide is pbo₂ (s) → pb (s) + o₂ (g), which is correctly balanced with one lead atom and two oxygen atoms on each side. Pbo2 = pb + o2. A balanced chemical equation often may be derived from a qualitative description of. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.slideserve.com
PPT Synthesis and Reactions PowerPoint Presentation Decomposition Of Lead Iv Oxide Balanced Equation A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. The balanced equation will appear above. Consider as an example the. The balanced chemical equation representing the decomposition of lead (iv) oxide is option a: The balanced chemical equation for the decomposition of lead. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.numerade.com
SOLVED Part Write a balanced equation for the of lead Decomposition Of Lead Iv Oxide Balanced Equation For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. Consider as an example the. See examples of decomposition reactions, such as. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. A balanced chemical equation often may be derived from a qualitative. Decomposition Of Lead Iv Oxide Balanced Equation.
From slideplayer.com
CHEMICAL REACTIONS Reactants Zn + I2 Product Zn I2. ppt download Decomposition Of Lead Iv Oxide Balanced Equation Consider as an example the. This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. The balanced chemical equation for the decomposition of lead (iv) oxide is pbo₂ (s) → pb (s) + o₂ (g), which is correctly balanced with one lead atom and two oxygen atoms on each side. The balanced chemical equation. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.chegg.com
Solved Consider the of lead(IV) oxide Decomposition Of Lead Iv Oxide Balanced Equation This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. Consider as an example the. The balanced equation will appear above. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. To balance a chemical equation, enter an. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.slideserve.com
PPT STOICHIOMETRY PowerPoint Presentation, free download ID312262 Decomposition Of Lead Iv Oxide Balanced Equation For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. Consider as an example the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will appear above. The balanced chemical equation representing the decomposition of lead (iv) oxide is. Decomposition Of Lead Iv Oxide Balanced Equation.
From slideplayer.com
Chapter 11 Review “Chemical Reactions” ppt download Decomposition Of Lead Iv Oxide Balanced Equation Consider as an example the. Pbo2 = pb + o2. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. The balanced chemical equation for the decomposition of lead iv oxide to yield lead ii oxide and a colorless gas is: See examples of decomposition reactions, such as. The balanced. Decomposition Of Lead Iv Oxide Balanced Equation.
From slideplayer.com
Chemical Reactions. ppt download Decomposition Of Lead Iv Oxide Balanced Equation The balanced chemical equation for the decomposition of lead iv oxide to yield lead ii oxide and a colorless gas is: See examples of decomposition reactions, such as. The balanced equation will appear above. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection.. Decomposition Of Lead Iv Oxide Balanced Equation.
From exoxkmyoc.blob.core.windows.net
Thermal Of Lead Iv Oxide at James Dewey blog Decomposition Of Lead Iv Oxide Balanced Equation A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. See examples of decomposition reactions, such as. The balanced chemical equation for the decomposition of lead (iv) oxide. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.slideserve.com
PPT Synthesis and Reactions PowerPoint Presentation Decomposition Of Lead Iv Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced chemical equation for the decomposition of lead iv oxide to yield lead ii oxide and a colorless gas is: The balanced chemical equation for the decomposition of lead (iv) oxide is pbo₂ (s) → pb (s) + o₂ (g), which is. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.numerade.com
Smelting When tin(IV) oxide is heated with carbon in a process called Decomposition Of Lead Iv Oxide Balanced Equation To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. See examples of decomposition reactions, such as. The balanced chemical equation representing the decomposition of lead (iv) oxide is option a: A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.numerade.com
SOLVEDA common demonstration in chemistry courses involves adding a Decomposition Of Lead Iv Oxide Balanced Equation The balanced chemical equation for the decomposition of lead iv oxide to yield lead ii oxide and a colorless gas is: Consider as an example the. The balanced equation will appear above. The balanced chemical equation for the decomposition of lead (iv) oxide is pbo₂ (s) → pb (s) + o₂ (g), which is correctly balanced with one lead atom. Decomposition Of Lead Iv Oxide Balanced Equation.
From slideplayer.com
Chemical Reactions. ppt download Decomposition Of Lead Iv Oxide Balanced Equation For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. The balanced equation will appear above. The balanced chemical equation representing the decomposition of lead (iv) oxide is option a: The balanced chemical equation for the decomposition of lead (iv) oxide is pbo₂ (s) → pb (s) + o₂ (g),. Decomposition Of Lead Iv Oxide Balanced Equation.
From slideplayer.com
Chemical Reactions and Collision Theory ppt download Decomposition Of Lead Iv Oxide Balanced Equation See examples of decomposition reactions, such as. Pbo2 = pb + o2. This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Consider as an example the. For each p b4+ we need two negative oxide ions. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.numerade.com
Lead (II) carbonate to form lead (II) oxide and carbon Decomposition Of Lead Iv Oxide Balanced Equation Consider as an example the. The balanced chemical equation representing the decomposition of lead (iv) oxide is option a: This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. See examples of decomposition reactions, such as. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The. Decomposition Of Lead Iv Oxide Balanced Equation.
From slideplayer.com
Synthesis Reactions Sodium metal reacts with chlorine gas ppt download Decomposition Of Lead Iv Oxide Balanced Equation This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. The balanced chemical equation for the decomposition of lead iv oxide to yield lead ii oxide and a colorless gas is: The balanced equation. Decomposition Of Lead Iv Oxide Balanced Equation.
From slideplayer.com
Chemical Reactions. ppt download Decomposition Of Lead Iv Oxide Balanced Equation The balanced chemical equation for the decomposition of lead (iv) oxide is pbo₂ (s) → pb (s) + o₂ (g), which is correctly balanced with one lead atom and two oxygen atoms on each side. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.numerade.com
SOLVED Lead metal is heated with oxygen in air to yield solid lead(IV Decomposition Of Lead Iv Oxide Balanced Equation The balanced chemical equation for the decomposition of lead iv oxide to yield lead ii oxide and a colorless gas is: This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. The balanced chemical equation representing the decomposition of lead (iv) oxide is option a: To balance a chemical equation, enter an equation of. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.pw.live
Lead IV Oxide Formula, Structure, Properties, Uses Decomposition Of Lead Iv Oxide Balanced Equation A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. The balanced chemical equation representing the decomposition of lead (iv) oxide is option a: For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge.. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.numerade.com
SOLVED Be able to identify reactants and products in a chemical Decomposition Of Lead Iv Oxide Balanced Equation This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. See examples of decomposition reactions, such as. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button.. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.numerade.com
write a balanced equation to represent the of lead(IV Decomposition Of Lead Iv Oxide Balanced Equation The balanced equation will appear above. Consider as an example the. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. The balanced chemical equation for the decomposition of lead iv oxide to yield. Decomposition Of Lead Iv Oxide Balanced Equation.
From slideplayer.com
Types of Reactions Including reaction prediction. ppt download Decomposition Of Lead Iv Oxide Balanced Equation The balanced equation will appear above. Consider as an example the. This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. See examples of decomposition reactions, such as. A balanced chemical equation often may. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.numerade.com
SOLVEDLead(IV) oxide is a strong oxidizing agent. For example, lead(IV Decomposition Of Lead Iv Oxide Balanced Equation The balanced chemical equation for the decomposition of lead (iv) oxide is pbo₂ (s) → pb (s) + o₂ (g), which is correctly balanced with one lead atom and two oxygen atoms on each side. The balanced chemical equation representing the decomposition of lead (iv) oxide is option a: Pbo2 = pb + o2. For each p b4+ we need. Decomposition Of Lead Iv Oxide Balanced Equation.
From slideplayer.com
Classifying Chemical Reactions ppt download Decomposition Of Lead Iv Oxide Balanced Equation For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. The balanced chemical equation representing the decomposition of lead (iv) oxide is option a: To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. A balanced chemical equation often may be derived from. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.coursehero.com
[Solved] What is the lewis ionic structure for Lead (IV) Oxide (PbO2 Decomposition Of Lead Iv Oxide Balanced Equation The balanced equation will appear above. The balanced chemical equation representing the decomposition of lead (iv) oxide is option a: See examples of decomposition reactions, such as. Pbo2 = pb + o2. This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. A balanced chemical equation often may be derived from a qualitative description. Decomposition Of Lead Iv Oxide Balanced Equation.
From slideplayer.com
Chemical Reactions & Equations ppt download Decomposition Of Lead Iv Oxide Balanced Equation This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. Consider as an example the. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Pbo2 = pb + o2. For each p b4+ we need two negative oxide ions o2− to balance out total positive and. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.numerade.com
SOLVED 1 Write and balance this chemical equation cadmium is used Decomposition Of Lead Iv Oxide Balanced Equation See examples of decomposition reactions, such as. Consider as an example the. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. The balanced chemical equation for the decomposition of lead (iv) oxide is pbo₂ (s) → pb (s) + o₂ (g), which is. Decomposition Of Lead Iv Oxide Balanced Equation.
From slideplayer.com
Chemical Reactions Occur in Predictable Ways ppt download Decomposition Of Lead Iv Oxide Balanced Equation Pbo2 = pb + o2. The balanced chemical equation for the decomposition of lead (iv) oxide is pbo₂ (s) → pb (s) + o₂ (g), which is correctly balanced with one lead atom and two oxygen atoms on each side. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge.. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.toppr.com
In the of lead (ll) nitrate to give lead (ll) oxide Decomposition Of Lead Iv Oxide Balanced Equation A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. See examples of decomposition reactions, such as. The balanced chemical equation representing the decomposition of lead (iv) oxide is option a: The balanced equation will appear above. For each p b4+ we need two. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.chegg.com
Solved Lead (IV) oxide dissolves in concentrated Decomposition Of Lead Iv Oxide Balanced Equation The balanced chemical equation for the decomposition of lead (iv) oxide is pbo₂ (s) → pb (s) + o₂ (g), which is correctly balanced with one lead atom and two oxygen atoms on each side. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. Pbo2 = pb + o2.. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.youtube.com
How to Balance Pb(NO3)2 = PbO + NO2 + O2 of Lead (II Decomposition Of Lead Iv Oxide Balanced Equation Consider as an example the. A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. This reaction suggests that lead iv oxide (pbo2) decomposes to form lead. Decomposition Of Lead Iv Oxide Balanced Equation.
From www.youtube.com
How to Write the Formula for Lead (IV) oxide YouTube Decomposition Of Lead Iv Oxide Balanced Equation This reaction suggests that lead iv oxide (pbo2) decomposes to form lead ii oxide (pbo) and. See examples of decomposition reactions, such as. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced chemical equation for the decomposition of lead iv oxide to yield lead ii oxide and a colorless gas. Decomposition Of Lead Iv Oxide Balanced Equation.
From brainly.in
c. Lead dioxide [lead (IV) oxide]= Lead monoxide + Oxygen balance Decomposition Of Lead Iv Oxide Balanced Equation Pbo2 = pb + o2. See examples of decomposition reactions, such as. To balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Consider as an example the. The balanced chemical equation for the decomposition of lead iv oxide to yield lead ii oxide and a colorless gas is: The balanced chemical equation representing. Decomposition Of Lead Iv Oxide Balanced Equation.