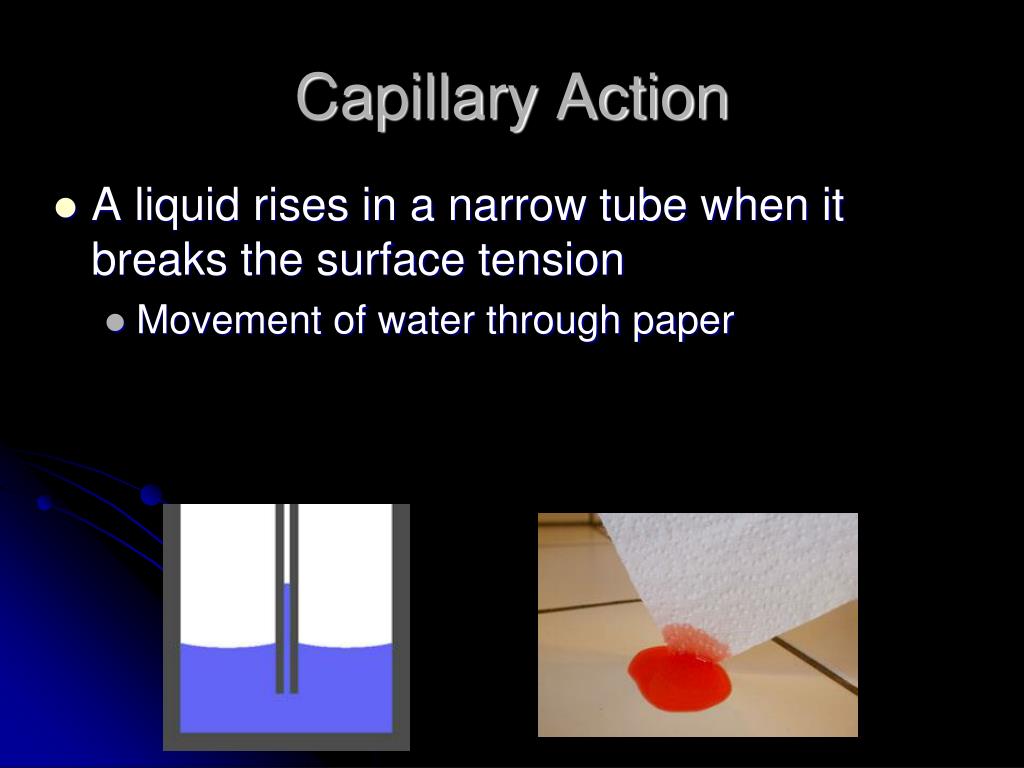
How Does Capillary Action Occur . Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. This movement does not require the force of gravity to occur. This primarily occurs due to adhesive and cohesive forces. The liquid is drawn upward due to this interaction between the phenomena. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures from substances. Capillary action is sometimes called capillary motion, capillarity, or wicking. In fact, it often acts in opposition to gravity. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules.
from www.slideserve.com
Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. The liquid is drawn upward due to this interaction between the phenomena. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures from substances. In fact, it often acts in opposition to gravity. This movement does not require the force of gravity to occur. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. Capillary action is sometimes called capillary motion, capillarity, or wicking. This primarily occurs due to adhesive and cohesive forces. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place.
PPT Liquids PowerPoint Presentation, free download ID2250045
How Does Capillary Action Occur Capillary action is sometimes called capillary motion, capillarity, or wicking. In fact, it often acts in opposition to gravity. This movement does not require the force of gravity to occur. The liquid is drawn upward due to this interaction between the phenomena. This primarily occurs due to adhesive and cohesive forces. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures from substances. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. Capillary action is sometimes called capillary motion, capillarity, or wicking.
From www.quirkyscience.com
Capillary Action from the Forces of Adhesion and Cohesion How Does Capillary Action Occur Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures from substances. The liquid is drawn upward due to this interaction between the phenomena. This movement does not require the force of. How Does Capillary Action Occur.
From www.researchgate.net
Principles of capillary action in drug loading and controlled release How Does Capillary Action Occur This movement does not require the force of gravity to occur. Capillary action is sometimes called capillary motion, capillarity, or wicking. In fact, it often acts in opposition to gravity. The liquid is drawn upward due to this interaction between the phenomena. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to. How Does Capillary Action Occur.
From wonderlab.org
Capillary Action WonderLab How Does Capillary Action Occur Capillary action is sometimes called capillary motion, capillarity, or wicking. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures from substances. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. The liquid is drawn upward due to this interaction between. How Does Capillary Action Occur.
From definitionklw.blogspot.com
Definition Of Capillary Action DEFINITION KLW How Does Capillary Action Occur This primarily occurs due to adhesive and cohesive forces. The liquid is drawn upward due to this interaction between the phenomena. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. In. How Does Capillary Action Occur.
From ar.inspiredpencil.com
Capillary Action How Does Capillary Action Occur This primarily occurs due to adhesive and cohesive forces. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. In fact, it often acts in opposition to gravity. A technique called thin layer. How Does Capillary Action Occur.
From www.slideserve.com
PPT CHAPTER 10 STATES OF MATTER PowerPoint Presentation, free How Does Capillary Action Occur Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. This movement does not require the force of gravity to occur. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures from substances. Capillary action is sometimes called capillary motion, capillarity, or. How Does Capillary Action Occur.
From www.youtube.com
Capillary action dissected YouTube How Does Capillary Action Occur In fact, it often acts in opposition to gravity. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. Capillary action occurs when the adhesion to the walls is stronger than the. How Does Capillary Action Occur.
From sciencetitlepage.wordpress.com
Capillary Action Science Title Page How Does Capillary Action Occur In fact, it often acts in opposition to gravity. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. This primarily occurs due to adhesive and cohesive forces. Capillary action is sometimes called capillary motion, capillarity, or wicking. The liquid is drawn upward due to this interaction between the phenomena.. How Does Capillary Action Occur.
From www.thoughtco.com
An Illustrated Guide to Capillary Fluid Exchange) How Does Capillary Action Occur This primarily occurs due to adhesive and cohesive forces. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. This movement does not require the force of gravity to occur. Capillary action is sometimes called capillary motion, capillarity, or wicking. Capillary action is defined as the spontaneous flow of a liquid. How Does Capillary Action Occur.
From socratic.org
Why does capillary action occur? + Example How Does Capillary Action Occur In fact, it often acts in opposition to gravity. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. This movement does not require the force of gravity to occur. This. How Does Capillary Action Occur.
From www.britannica.com
Capillary anatomy Britannica How Does Capillary Action Occur In fact, it often acts in opposition to gravity. The liquid is drawn upward due to this interaction between the phenomena. This primarily occurs due to adhesive and cohesive forces. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures from substances. Capillary action is defined as the spontaneous flow. How Does Capillary Action Occur.
From www.biolinscientific.com
Capillary action how contact angle and surface tension are related? How Does Capillary Action Occur Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. The liquid is drawn upward due to this interaction between the phenomena. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures from substances. Capillary action occurs when the adhesion to the. How Does Capillary Action Occur.
From www.thoughtco.com
What Is Capillary Action? Definition and Examples How Does Capillary Action Occur The liquid is drawn upward due to this interaction between the phenomena. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures from substances. Capillary action occurs when the adhesion to the. How Does Capillary Action Occur.
From slideplayer.com
Roots, Stems and Leaves Part ppt download How Does Capillary Action Occur This movement does not require the force of gravity to occur. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. In fact, it often acts in opposition to gravity. Capillary action. How Does Capillary Action Occur.
From teamgrecoatlakeview.blogspot.com
Capillary Action In Plants Plants BY How Does Capillary Action Occur In fact, it often acts in opposition to gravity. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures from substances. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. This primarily occurs due to adhesive and cohesive forces. This movement. How Does Capillary Action Occur.
From ar.inspiredpencil.com
Capillary Action Definition How Does Capillary Action Occur We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures from substances. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid. How Does Capillary Action Occur.
From www.slideserve.com
PPT Liquids PowerPoint Presentation, free download ID2250045 How Does Capillary Action Occur Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. This movement does not require the force of gravity to occur. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. We can define capillary action as a phenomenon where the ascension of. How Does Capillary Action Occur.
From eduinput.com
What are Capillaries?Types, Mechanism of Action, and Functions How Does Capillary Action Occur A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures from substances. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. This primarily occurs due to adhesive and cohesive forces. Capillary action is sometimes called capillary motion, capillarity, or wicking.. How Does Capillary Action Occur.
From www.youtube.com
Capillary Action YouTube How Does Capillary Action Occur In fact, it often acts in opposition to gravity. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. This movement does not require the force of gravity to occur. A technique. How Does Capillary Action Occur.
From basementsystemusa.com
Capillary Action Explained Understanding Moisture Movement in Leaky How Does Capillary Action Occur This movement does not require the force of gravity to occur. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. In fact, it often acts in opposition to gravity. Capillary action is sometimes called capillary motion, capillarity, or wicking. The liquid is drawn upward due to this interaction between. How Does Capillary Action Occur.
From www.quirkyscience.com
Capillary Action from the Forces of Adhesion and Cohesion How Does Capillary Action Occur Capillary action is sometimes called capillary motion, capillarity, or wicking. Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. The liquid is drawn upward due to this interaction between the phenomena. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. A. How Does Capillary Action Occur.
From www.slideserve.com
PPT Hydrology PowerPoint Presentation, free download ID2957929 How Does Capillary Action Occur We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. This movement does not require the force of gravity to occur. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures from substances. This primarily occurs due to adhesive and. How Does Capillary Action Occur.
From definitionklw.blogspot.com
Definition Of Capillary Action DEFINITION KLW How Does Capillary Action Occur This movement does not require the force of gravity to occur. In fact, it often acts in opposition to gravity. This primarily occurs due to adhesive and cohesive forces. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. Capillary action occurs when the adhesion to the walls is stronger. How Does Capillary Action Occur.
From sciencenotes.org
Capillary Action What It Is and How It Works How Does Capillary Action Occur We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. This movement does not require the force of gravity to occur. In fact, it often acts in opposition to gravity. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures. How Does Capillary Action Occur.
From www.youtube.com
Capillary Action YouTube How Does Capillary Action Occur In fact, it often acts in opposition to gravity. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. This movement does not require the force of gravity to occur. Capillary action. How Does Capillary Action Occur.
From www.ddcoatings.co.uk
What is Capillary Action? How Does Capillary Action Occur Capillary action is sometimes called capillary motion, capillarity, or wicking. In fact, it often acts in opposition to gravity. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. A technique called. How Does Capillary Action Occur.
From study.com
Capillary Action in Plants Definition & Examples Video & Lesson How Does Capillary Action Occur Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures from substances. This movement does not require the force of gravity to occur. Capillary action occurs when the adhesion to the walls. How Does Capillary Action Occur.
From www.popoptiq.com
3 Types of Capillaries (Plus Interesting Facts) How Does Capillary Action Occur Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. This movement does not require the force of gravity to occur. In fact, it often acts in opposition to gravity. This primarily. How Does Capillary Action Occur.
From www.ddcoatings.co.uk
What is Capillary Action? How Does Capillary Action Occur Capillary action is sometimes called capillary motion, capillarity, or wicking. The liquid is drawn upward due to this interaction between the phenomena. In fact, it often acts in opposition to gravity. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. This primarily occurs due to adhesive and cohesive forces. We can. How Does Capillary Action Occur.
From chem.libretexts.org
Capillary Action Chemistry LibreTexts How Does Capillary Action Occur We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. This primarily occurs due to adhesive and cohesive forces. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. The liquid is drawn upward due to this interaction between the phenomena. Capillary. How Does Capillary Action Occur.
From www.slideserve.com
PPT The Nature of Matter PowerPoint Presentation, free download ID How Does Capillary Action Occur The liquid is drawn upward due to this interaction between the phenomena. This movement does not require the force of gravity to occur. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. This primarily occurs due to adhesive and cohesive forces. A technique called thin layer chromatography uses capillary action in. How Does Capillary Action Occur.
From study.com
Capillary Fluid Exchange Overview & Hydrostatic Pressure Video How Does Capillary Action Occur This primarily occurs due to adhesive and cohesive forces. The liquid is drawn upward due to this interaction between the phenomena. In fact, it often acts in opposition to gravity. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to separate mixtures from substances. Capillary action occurs when the adhesion to the. How Does Capillary Action Occur.
From www.slideserve.com
PPT Physics 221, March 15 PowerPoint Presentation, free download ID How Does Capillary Action Occur This movement does not require the force of gravity to occur. Capillary action is sometimes called capillary motion, capillarity, or wicking. This primarily occurs due to adhesive and cohesive forces. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. In fact, it often acts in opposition to gravity. Capillary action occurs. How Does Capillary Action Occur.
From www.lecturio.com
Capillaries Histology Concise Medical Knowledge How Does Capillary Action Occur Capillary action occurs when the adhesion to the walls is stronger than the cohesive forces between the liquid molecules. The liquid is drawn upward due to this interaction between the phenomena. This movement does not require the force of gravity to occur. A technique called thin layer chromatography uses capillary action in which a layer of liquid is used to. How Does Capillary Action Occur.
From lewis-has-ritter.blogspot.com
Capillary Action Is a Term Used to Describe How LewishasRitter How Does Capillary Action Occur We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. Capillary action is defined as the spontaneous flow of a liquid into a narrow tube or porous material. In fact, it often acts in opposition to gravity. Capillary action is sometimes called capillary motion, capillarity, or wicking. A technique called. How Does Capillary Action Occur.