Delineate Trial . Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on. All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. Efficacy and toxicity in the delineate. Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions.
from www.semanticscholar.org
All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Efficacy and toxicity in the delineate. To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer:
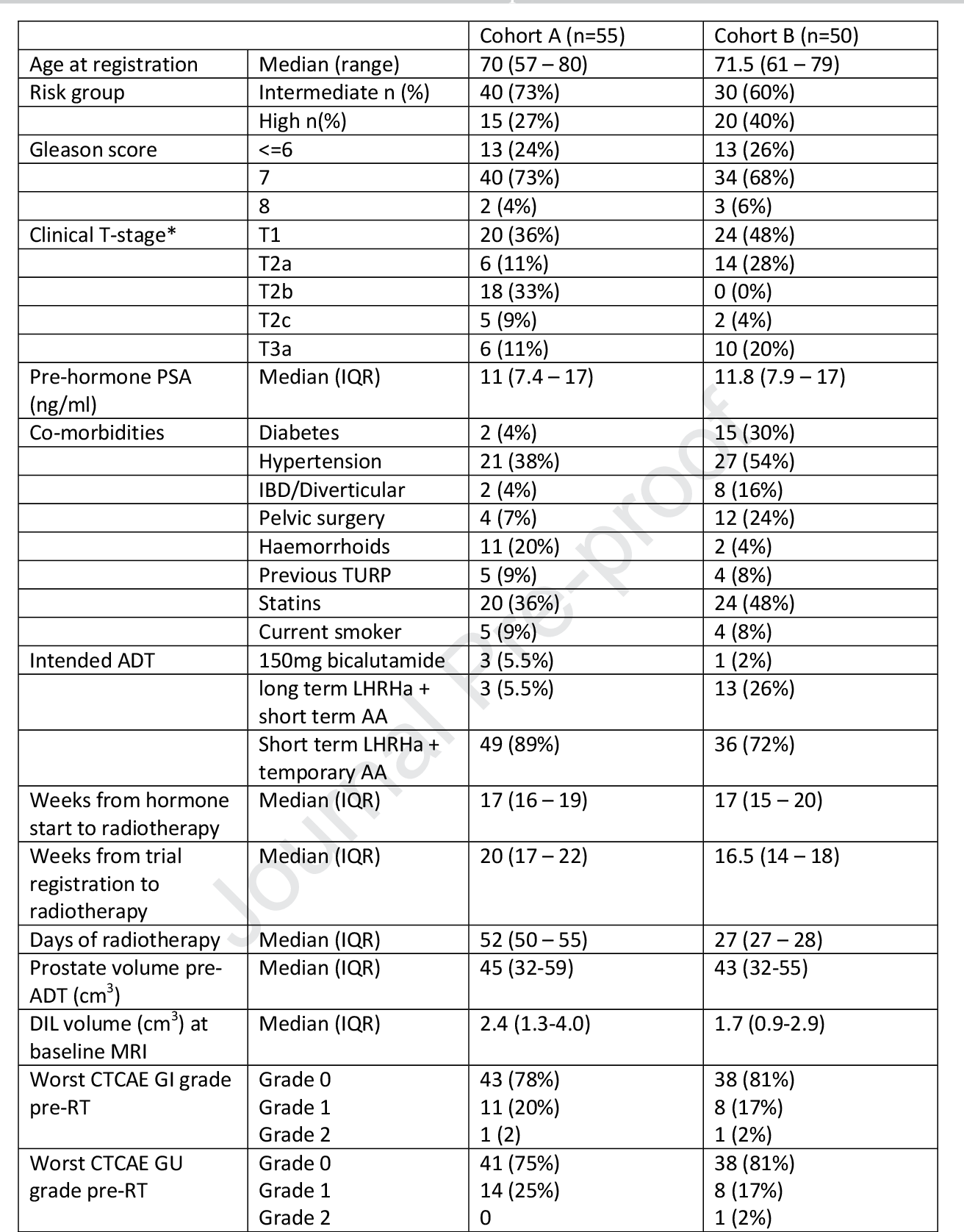
Table 1 from Journal Preproof Standard and hypofractionated dose
Delineate Trial Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: Efficacy and toxicity in the delineate. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20.
From www.researchgate.net
Treatmentnonspecific effects in clinical trials. (a) Hypothetical Delineate Trial Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: Efficacy and toxicity in the delineate. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose escalation to intraprostatic. Delineate Trial.
From www.redjournal.org
Standard and Hypofractionated Dose Escalation to Intraprostatic Tumor Delineate Trial To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on. Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20. Delineate Trial.
From www.redjournal.org
Standard and Hypofractionated Dose Escalation to Intraprostatic Tumor Delineate Trial Efficacy and toxicity in the delineate. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose. Delineate Trial.
From www.redjournal.org
Standard and Hypofractionated Dose Escalation to Intraprostatic Tumor Delineate Trial To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Efficacy and toxicity in the delineate. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Standard and hypofractionated dose. Delineate Trial.
From www.researchgate.net
DRN 5HT photostimulation inhibits aPC activity during odor Delineate Trial Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Efficacy and toxicity in the delineate. To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on. Standard and hypofractionated dose. Delineate Trial.
From www.theinsaneapp.com
Delineate Delineate Trial To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on. All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer:. Delineate Trial.
From aigems.net
Delineate Delineate Trial The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Efficacy and toxicity in the delineate. All patients within the delineate trial received. Delineate Trial.
From www.redjournal.org
Standard and Hypofractionated Dose Escalation to Intraprostatic Tumor Delineate Trial Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated. Delineate Trial.
From www.researchgate.net
Cropping system A) stability and B) drought resilience versus relative Delineate Trial Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Efficacy and toxicity in the delineate. The delineate trial has shown safety, tolerability, and feasibility of focal. Delineate Trial.
From apprater.net
Delineate Machine learning predictive analytics made easy is on AppRater Delineate Trial All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on.. Delineate Trial.
From www.istockphoto.com
Describe Delineate Stock Illustration Download Image Now Explaining Delineate Trial The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Efficacy and toxicity in the delineate. To report a planned analysis of the. Delineate Trial.
From delineate.ai
Delineate About Us Your Consumers Live and Direct Delineate Trial Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in. Delineate Trial.
From www.aishowcase.io
Delineate AI Showcase Delineate Trial The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. Efficacy and toxicity in the delineate. To report a planned analysis of the efficacy and toxicity. Delineate Trial.
From www.semanticscholar.org
Table 1 from Journal Preproof Standard and hypofractionated dose Delineate Trial Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. Standard and hypofractionated. Delineate Trial.
From www.redjournal.org
Standard and Hypofractionated Dose Escalation to Intraprostatic Tumor Delineate Trial Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Efficacy and toxicity in the delineate. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised. Delineate Trial.
From slideplayer.com
Withholding and Withdrawing in critical dialysis 蔡壁如. ppt download Delineate Trial All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Efficacy and toxicity in the delineate. Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: Standard. Delineate Trial.
From www.researchgate.net
Study design. (A). Within IMRT clinical trial (CRC1766) 612 Delineate Trial Efficacy and toxicity in the delineate. All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. To report a planned analysis of the efficacy and toxicity. Delineate Trial.
From www.researchgate.net
(A) International Prostate Symptom Score (IPSS) total score median and Delineate Trial To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on. Efficacy and toxicity in the delineate. Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose. Delineate Trial.
From www.researchgate.net
2D image of LA. Twodimensional image illustrating how to delineate Delineate Trial To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on. Efficacy and toxicity in the delineate. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. All patients within the. Delineate Trial.
From www.thaka.io
Delineate Developer Tools Explore 10,000+ AI Tools & Explore Best Delineate Trial Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: Efficacy and toxicity in the delineate. All patients within the delineate trial received radiation therapy to the whole prostate, to a. Delineate Trial.
From www.researchgate.net
Study design. (A). Within IMRT clinical trial (CRC1766) 612 Delineate Trial The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. To report a planned analysis of the efficacy and toxicity of dose escalation. Delineate Trial.
From slideplayer.com
Volume 18, Issue 3, Pages (January 2017) ppt download Delineate Trial Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on.. Delineate Trial.
From thecontentauthority.com
Delineate vs Differentiate Meaning And Differences Delineate Trial All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. Efficacy and toxicity in the delineate. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in. Delineate Trial.
From www.insightplatforms.com
Platform Demo Delineate Insight Platforms Delineate Trial The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Standard and hypofractionated. Delineate Trial.
From www.delineate.co
Delineate Predictive Analytics Delineate Trial To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on. All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in. Delineate Trial.
From www.researchgate.net
(PDF) Standard and Hypofractionated Dose Escalation to Intraprostatic Delineate Trial The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. Standard and hypofractionated. Delineate Trial.
From www.redjournal.org
PSMAPET and MRIBased Focal Dose Escalated Radiation Therapy of Delineate Trial All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic. Delineate Trial.
From top.aibase.com
Delineate 解锁数据潜力,提供智能预测分析解决方案 Delineate Trial Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions.. Delineate Trial.
From tintopress.com
Delineate Tinto Press Delineate Trial All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. Efficacy and toxicity in the delineate. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Standard. Delineate Trial.
From thecontentauthority.com
Delineate vs Demarcate When And How Can You Use Each One? Delineate Trial Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Efficacy and toxicity in. Delineate Trial.
From www.ai-navigate.com
Delineate Delineate Trial The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Efficacy and toxicity in the delineate. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: All patients within the delineate trial received. Delineate Trial.
From ai.cryptobk.jp
【データ分析】AIツール「Delineate」の機能や使い方・メリットを詳しく解説 AI ナビ Delineate Trial All patients within the delineate trial received radiation therapy to the whole prostate, to a dose of 74 gy in 37 fractions and 60 gy in 20. To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer:. Delineate Trial.
From www.researchgate.net
(PDF) Development of a Measure to Delineate the Clinical Trials Nursing Delineate Trial To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Efficacy and toxicity in the delineate. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. The delineate trial has. Delineate Trial.
From www.ctsnet.org
AATS Clinical Trials Methods Course Delineate Trial Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in localized prostate cancer: Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: Efficacy and toxicity in. Delineate Trial.
From www.redjournal.org
Standard and Hypofractionated Dose Escalation to Intraprostatic Tumor Delineate Trial Standard and hypofractionated dose escalation to intraprostatic tumour nodules in localised prostate cancer: To report a planned analysis of the efficacy and toxicity of dose escalation to the intraprostatic dominant nodule identified on. The delineate trial has shown safety, tolerability, and feasibility of focal boosting in 20 or 37 fractions. Standard and hypofractionated dose escalation to intraprostatic tumor nodules in. Delineate Trial.