Dilution Factor Of Undiluted Solution . The formula to calculate dilution factor concentration is simple yet powerful: Df = vf / vi. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. The sample concentration is calculated as: Vf = final volume of the diluted solution. Df = v f v i = 10.0ml 0.1ml = 100. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. If you do not have the standard, you can find out. Dilution factor (df) = final volume (vf) / initial. Effect of dilution factor on concentration. The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution. Remember, dilution factor tells us the ratio of the initial (undiluted) volume of solution to the final. The formula used to calculate the dilution factor is as follows:
from www.youtube.com
The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Effect of dilution factor on concentration. Remember, dilution factor tells us the ratio of the initial (undiluted) volume of solution to the final. The formula used to calculate the dilution factor is as follows: The sample concentration is calculated as: If you do not have the standard, you can find out. Vf = final volume of the diluted solution. Df = vf / vi. Df = v f v i = 10.0ml 0.1ml = 100.
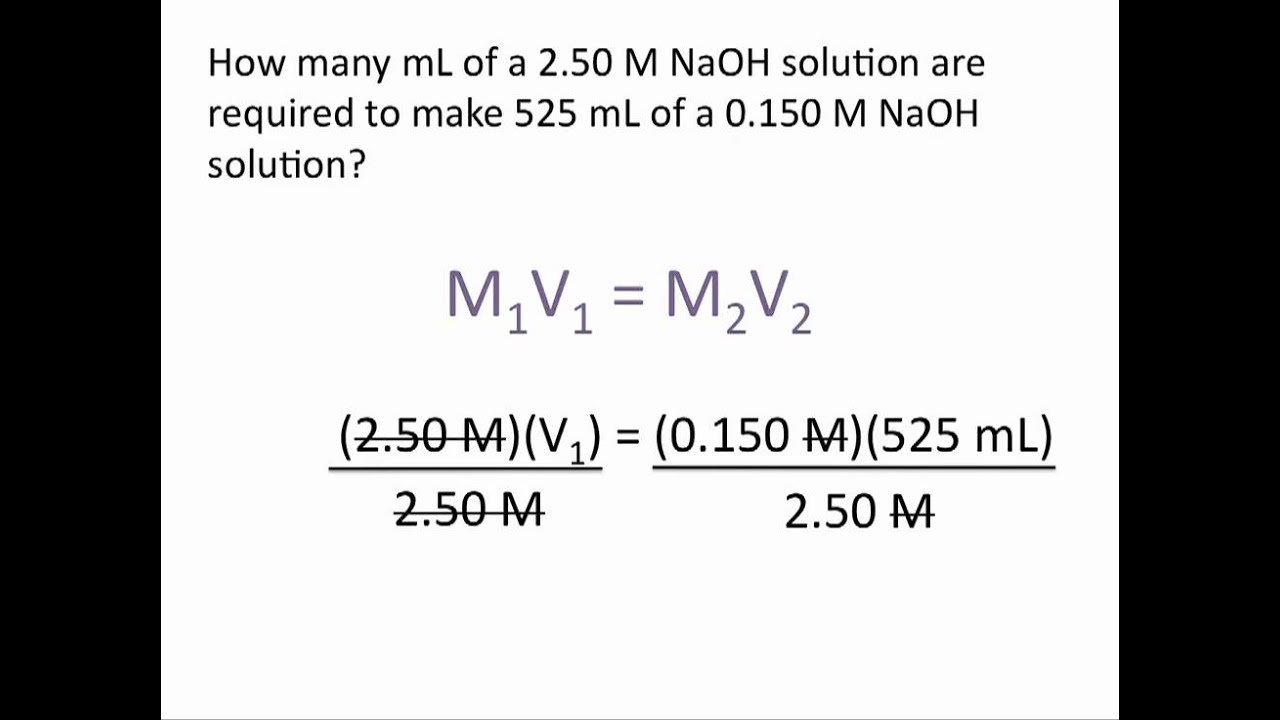
Dilution Problems Chemistry Tutorial YouTube
Dilution Factor Of Undiluted Solution Effect of dilution factor on concentration. Dilution factor (df) = final volume (vf) / initial. The formula used to calculate the dilution factor is as follows: Vf = final volume of the diluted solution. Df = vf / vi. Df = v f v i = 10.0ml 0.1ml = 100. The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution. The formula to calculate dilution factor concentration is simple yet powerful: Remember, dilution factor tells us the ratio of the initial (undiluted) volume of solution to the final. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. The sample concentration is calculated as: The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. If you do not have the standard, you can find out. Effect of dilution factor on concentration.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Factor Of Undiluted Solution Effect of dilution factor on concentration. Df = v f v i = 10.0ml 0.1ml = 100. Vf = final volume of the diluted solution. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution. Dilution Factor Of Undiluted Solution.
From www.slideserve.com
PPT Chapter 13 PowerPoint Presentation, free download ID5812279 Dilution Factor Of Undiluted Solution V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Dilution factor (df) = final volume (vf) / initial. The formula used to calculate the dilution factor is as follows: If you do not have the standard, you can find out. Df = v f v i = 10.0ml 0.1ml = 100. Df =. Dilution Factor Of Undiluted Solution.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Factor Of Undiluted Solution Vf = final volume of the diluted solution. Effect of dilution factor on concentration. The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution. Remember, dilution factor tells us the ratio of the initial (undiluted) volume of solution to the final. Df = vf /. Dilution Factor Of Undiluted Solution.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY Dilution Factor Of Undiluted Solution The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. The sample concentration is calculated as: Df = v f v i = 10.0ml 0.1ml = 100. Dilution factor (df) = final volume (vf) / initial. The formula used to calculate the dilution factor is as. Dilution Factor Of Undiluted Solution.
From general.chemistrysteps.com
Dilution of a Stock Solution and Calculations Based Morality Dilution Factor Of Undiluted Solution The formula used to calculate the dilution factor is as follows: The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Df = vf / vi. Dilution factor (df) = final volume (vf) / initial. The answer is basically correct (see note at the end of. Dilution Factor Of Undiluted Solution.
From www.slideserve.com
PPT Concentration and Dilution of Solutions PowerPoint Presentation Dilution Factor Of Undiluted Solution The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution. If you do not have the standard, you can find out. The formula to calculate dilution factor concentration is simple yet powerful: Vf = final volume of the diluted solution. Remember, dilution factor tells us. Dilution Factor Of Undiluted Solution.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Factor Of Undiluted Solution The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. If you do not have the standard, you can find out. The formula to calculate dilution factor concentration is simple yet powerful: The sample concentration is calculated as: Df = vf / vi. Effect of dilution. Dilution Factor Of Undiluted Solution.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Factor Of Undiluted Solution Dilution factor (df) = final volume (vf) / initial. Effect of dilution factor on concentration. The formula used to calculate the dilution factor is as follows: V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Remember, dilution factor tells us the ratio of the initial (undiluted) volume of solution to the final. The. Dilution Factor Of Undiluted Solution.
From support.axionbio.com
Understanding of Cell Sample Dilution Factor Dilution Factor Of Undiluted Solution Df = vf / vi. If you do not have the standard, you can find out. The sample concentration is calculated as: The formula used to calculate the dilution factor is as follows: Dilution factor (df) = final volume (vf) / initial. The formula to calculate dilution factor concentration is simple yet powerful: The answer is basically correct (see note. Dilution Factor Of Undiluted Solution.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Factor Of Undiluted Solution The formula to calculate dilution factor concentration is simple yet powerful: V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Dilution factor (df) = final volume (vf) / initial. The formula used to calculate the dilution factor is as follows: If you do not have the standard, you can find out. Df =. Dilution Factor Of Undiluted Solution.
From ar.inspiredpencil.com
Dilute Science Dilution Factor Of Undiluted Solution The formula used to calculate the dilution factor is as follows: The formula to calculate dilution factor concentration is simple yet powerful: Df = vf / vi. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Remember, dilution factor tells us the ratio of the initial (undiluted) volume of solution to the final.. Dilution Factor Of Undiluted Solution.
From www.pinterest.com
Dilution when solvent is added to dilute a solution, the number of Dilution Factor Of Undiluted Solution Df = vf / vi. The sample concentration is calculated as: The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total.. Dilution Factor Of Undiluted Solution.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Factor Of Undiluted Solution Df = vf / vi. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution. Vf = final volume of the diluted solution. Remember, dilution factor tells us the. Dilution Factor Of Undiluted Solution.
From www.numerade.com
SOLVED Part 1 See Periodic Table See Hint Match the dilution with the Dilution Factor Of Undiluted Solution V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Dilution factor (df) = final volume (vf) / initial. The formula used to calculate the dilution factor is as follows:. Dilution Factor Of Undiluted Solution.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Factor Of Undiluted Solution Effect of dilution factor on concentration. The formula used to calculate the dilution factor is as follows: Dilution factor (df) = final volume (vf) / initial. Df = vf / vi. The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution. The formula to calculate. Dilution Factor Of Undiluted Solution.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Dilution Factor Of Undiluted Solution Dilution factor (df) = final volume (vf) / initial. If you do not have the standard, you can find out. The formula to calculate dilution factor concentration is simple yet powerful: The sample concentration is calculated as: Vf = final volume of the diluted solution. Effect of dilution factor on concentration. Df = v f v i = 10.0ml 0.1ml. Dilution Factor Of Undiluted Solution.
From www.slideserve.com
PPT Serial Dilution PowerPoint Presentation, free download ID3119682 Dilution Factor Of Undiluted Solution The formula used to calculate the dilution factor is as follows: The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution. Effect of dilution factor on concentration. Remember, dilution factor tells us the ratio of the initial (undiluted) volume of solution to the final. Df. Dilution Factor Of Undiluted Solution.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Dilution Factor Of Undiluted Solution The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Vf = final volume of the diluted solution. Effect of dilution factor on concentration. The formula to calculate dilution. Dilution Factor Of Undiluted Solution.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution Factor Of Undiluted Solution The sample concentration is calculated as: Effect of dilution factor on concentration. Dilution factor (df) = final volume (vf) / initial. The formula used to calculate the dilution factor is as follows: Vf = final volume of the diluted solution. Df = v f v i = 10.0ml 0.1ml = 100. Df = vf / vi. V f = aliquot. Dilution Factor Of Undiluted Solution.
From www.transtutors.com
(Solved) Match The Dilution With The Correct Solution Shown In The Dilution Factor Of Undiluted Solution Effect of dilution factor on concentration. The sample concentration is calculated as: If you do not have the standard, you can find out. Dilution factor (df) = final volume (vf) / initial. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Remember, dilution factor tells us the ratio of the initial (undiluted) volume. Dilution Factor Of Undiluted Solution.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Factor Of Undiluted Solution The formula used to calculate the dilution factor is as follows: V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Vf = final volume of the diluted solution. Df. Dilution Factor Of Undiluted Solution.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Factor Of Undiluted Solution Remember, dilution factor tells us the ratio of the initial (undiluted) volume of solution to the final. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. If you do. Dilution Factor Of Undiluted Solution.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID Dilution Factor Of Undiluted Solution The answer is basically correct (see note at the end of my answer about significant figures) but there is a simpler method, using a dilution. Dilution factor (df) = final volume (vf) / initial. The formula to calculate dilution factor concentration is simple yet powerful: Effect of dilution factor on concentration. The formula used to calculate the dilution factor is. Dilution Factor Of Undiluted Solution.
From www.numerade.com
SOLVED To calculate the real concentration of an undiluted solution Dilution Factor Of Undiluted Solution Remember, dilution factor tells us the ratio of the initial (undiluted) volume of solution to the final. The sample concentration is calculated as: Df = vf / vi. The formula used to calculate the dilution factor is as follows: The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present. Dilution Factor Of Undiluted Solution.
From socratic.org
How can I calculate the dilution factor using concentration? Socratic Dilution Factor Of Undiluted Solution Dilution factor (df) = final volume (vf) / initial. Vf = final volume of the diluted solution. Df = vf / vi. The formula to calculate dilution factor concentration is simple yet powerful: The formula used to calculate the dilution factor is as follows: V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml.. Dilution Factor Of Undiluted Solution.
From www.youtube.com
How to Calculate Dilution Factor YouTube Dilution Factor Of Undiluted Solution The sample concentration is calculated as: Df = vf / vi. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. The formula used to calculate the dilution factor is as follows: V f = aliquot volume + diluent volume = (0.1 + 9.9) ml =. Dilution Factor Of Undiluted Solution.
From www.chegg.com
Solved 1. Complete the following dilution series Transfer Dilution Factor Of Undiluted Solution Vf = final volume of the diluted solution. Effect of dilution factor on concentration. Dilution factor (df) = final volume (vf) / initial. If you do not have the standard, you can find out. Df = v f v i = 10.0ml 0.1ml = 100. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0. Dilution Factor Of Undiluted Solution.
From www.numerade.com
SOLVED Alternative way to solve dilution problems is to calculate the Dilution Factor Of Undiluted Solution The formula used to calculate the dilution factor is as follows: V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Effect of dilution factor on concentration. Df = v f v i = 10.0ml 0.1ml = 100. Df = vf / vi. The dilution factor (or dilution ratio) is the notation used to. Dilution Factor Of Undiluted Solution.
From www.chegg.com
Solved OD Extrapol. Mg/ml Dilution Factor 1 2 4 + UNKNOWN Dilution Factor Of Undiluted Solution Df = vf / vi. Remember, dilution factor tells us the ratio of the initial (undiluted) volume of solution to the final. Vf = final volume of the diluted solution. Dilution factor (df) = final volume (vf) / initial. The formula to calculate dilution factor concentration is simple yet powerful: Effect of dilution factor on concentration. The dilution factor (or. Dilution Factor Of Undiluted Solution.
From www.chegg.com
Solved You perform a serial dilution as shown in the figure Dilution Factor Of Undiluted Solution Dilution factor (df) = final volume (vf) / initial. V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Df = vf / vi. Df = v f v i = 10.0ml 0.1ml = 100. Vf = final volume of the diluted solution. The answer is basically correct (see note at the end of. Dilution Factor Of Undiluted Solution.
From www.slideshare.net
Serial dilution Dilution Factor Of Undiluted Solution The sample concentration is calculated as: The formula used to calculate the dilution factor is as follows: Df = vf / vi. Effect of dilution factor on concentration. Vf = final volume of the diluted solution. If you do not have the standard, you can find out. Remember, dilution factor tells us the ratio of the initial (undiluted) volume of. Dilution Factor Of Undiluted Solution.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration Dilution Factor Of Undiluted Solution The sample concentration is calculated as: If you do not have the standard, you can find out. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Dilution factor (df) = final volume (vf) / initial. The formula to calculate dilution factor concentration is simple yet. Dilution Factor Of Undiluted Solution.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution Factor Of Undiluted Solution The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. Df = v f v i = 10.0ml 0.1ml = 100. If you do not have the standard, you can find out. Effect of dilution factor on concentration. V f = aliquot volume + diluent volume. Dilution Factor Of Undiluted Solution.
From bdaschools.weebly.com
bdaschools Blog Dilution Factor Of Undiluted Solution V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. Effect of dilution factor on concentration. The formula used to calculate the dilution factor is as follows: Df = v f v i = 10.0ml 0.1ml = 100. Dilution factor (df) = final volume (vf) / initial. The sample concentration is calculated as: If. Dilution Factor Of Undiluted Solution.
From www.chegg.com
Solved Dilutions/Titers 25. Calculate the dilution factor Dilution Factor Of Undiluted Solution V f = aliquot volume + diluent volume = (0.1 + 9.9) ml = 10.0 ml. The dilution factor (or dilution ratio) is the notation used to express how much of the original stock solution is present in the total. The sample concentration is calculated as: The formula used to calculate the dilution factor is as follows: Remember, dilution factor. Dilution Factor Of Undiluted Solution.