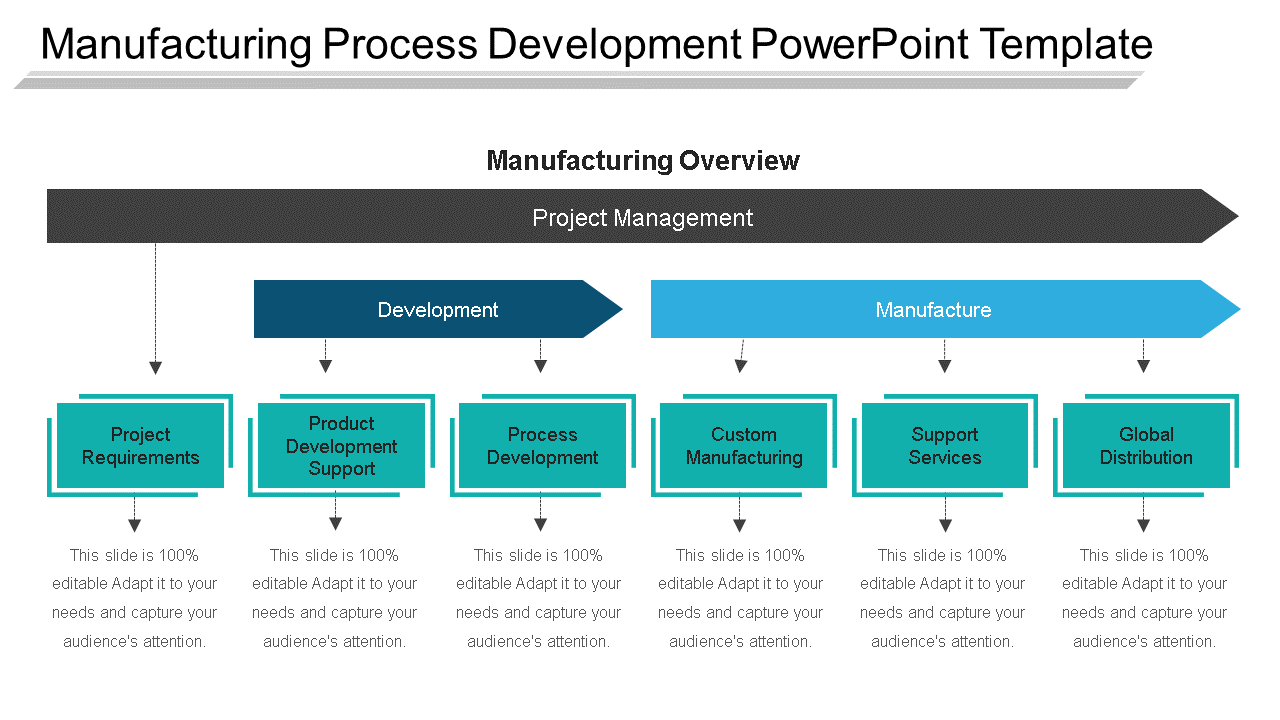
Manufacturing Process Qualification . A who guide to good manufacturing practice (gmp) requirements. Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. Validate the process to ensure that the process consistently deliver quality products. Process validation is a critical part of quality assurance in the manufacturing industry. The commercial manufacturing process is defined during this stage based on knowledge gained through. Since 2011 the fda recommends a lifecycle approach to process validation, including three. Its purpose is to ensure ongoing product quality. It involves the collection and analysis of data to ensure that a process consistently produces. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification.
from www.slideteam.net
It involves the collection and analysis of data to ensure that a process consistently produces. The commercial manufacturing process is defined during this stage based on knowledge gained through. Since 2011 the fda recommends a lifecycle approach to process validation, including three. Validate the process to ensure that the process consistently deliver quality products. A who guide to good manufacturing practice (gmp) requirements. Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. Its purpose is to ensure ongoing product quality. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. Process validation is a critical part of quality assurance in the manufacturing industry.
Top 10 Manufacturing Process Flow Charts With Templates, Samples and
Manufacturing Process Qualification Its purpose is to ensure ongoing product quality. It involves the collection and analysis of data to ensure that a process consistently produces. Since 2011 the fda recommends a lifecycle approach to process validation, including three. Process validation is a critical part of quality assurance in the manufacturing industry. A who guide to good manufacturing practice (gmp) requirements. Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. Its purpose is to ensure ongoing product quality. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. Validate the process to ensure that the process consistently deliver quality products. The commercial manufacturing process is defined during this stage based on knowledge gained through.
From www.yichyi.com
製程確效 Process Validation YICHYI Injection Molding Expert Manufacturing Process Qualification Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. Validate the process to ensure that the process consistently deliver quality products. A who guide to good manufacturing practice (gmp) requirements. It involves the collection and analysis of data to ensure that a process consistently produces. Its purpose is to ensure ongoing product. Manufacturing Process Qualification.
From www.mdpi.com
Applied Sciences Free FullText Development of a Procedure for Risk Manufacturing Process Qualification Validate the process to ensure that the process consistently deliver quality products. Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. A who guide to good manufacturing practice (gmp) requirements. Its purpose is to ensure ongoing product quality. The commercial manufacturing process is defined during this stage based on knowledge gained through.. Manufacturing Process Qualification.
From www.slideteam.net
Manufacturing Process Flow Chart Of Various Operation PPT Slide Manufacturing Process Qualification The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. Since 2011 the fda recommends a lifecycle approach to process validation, including three. The commercial manufacturing process is defined during this stage based on knowledge gained through. Process validation is a critical part of quality assurance in the manufacturing industry. Validate the process to ensure. Manufacturing Process Qualification.
From www.edrawsoft.com
Understanding Manufacturing Process Flowcharts (With Examples) Manufacturing Process Qualification Since 2011 the fda recommends a lifecycle approach to process validation, including three. It involves the collection and analysis of data to ensure that a process consistently produces. The commercial manufacturing process is defined during this stage based on knowledge gained through. A who guide to good manufacturing practice (gmp) requirements. Its purpose is to ensure ongoing product quality. The. Manufacturing Process Qualification.
From techqualitypedia.com
Manufacturing Processes Types Production Processes Types Manufacturing Process Qualification Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. The commercial manufacturing process is defined during this stage based on knowledge gained through. Validate the process to ensure that the process consistently deliver quality products. It involves the collection and analysis of data to ensure that a process consistently produces. The process. Manufacturing Process Qualification.
From www.edrawsoft.com
Understanding Manufacturing Process Flowcharts (With Examples) Manufacturing Process Qualification The commercial manufacturing process is defined during this stage based on knowledge gained through. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. Validate the process to ensure that the process consistently deliver quality products. Process validation is a critical part of quality assurance in the manufacturing industry. It involves the collection and analysis. Manufacturing Process Qualification.
From www.slideteam.net
Top 10 Manufacturing Process Flow Charts With Templates, Samples and Manufacturing Process Qualification A who guide to good manufacturing practice (gmp) requirements. Process validation is a critical part of quality assurance in the manufacturing industry. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. Its purpose is to ensure ongoing product quality. Establishing by key objective evidence that all key aspects of the process equipment and ancillary. Manufacturing Process Qualification.
From www.slideteam.net
Quality Control Process Flowchart For Manufacturing Company PPT Example Manufacturing Process Qualification The commercial manufacturing process is defined during this stage based on knowledge gained through. Its purpose is to ensure ongoing product quality. A who guide to good manufacturing practice (gmp) requirements. Process validation is a critical part of quality assurance in the manufacturing industry. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. Validate. Manufacturing Process Qualification.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and Manufacturing Process Qualification Since 2011 the fda recommends a lifecycle approach to process validation, including three. It involves the collection and analysis of data to ensure that a process consistently produces. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation.. Manufacturing Process Qualification.
From engineeringproductdesign.com
What are Manufacturing processes and their applications Manufacturing Process Qualification Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. Its purpose is to ensure ongoing product quality. A who guide to good manufacturing practice (gmp) requirements. The commercial manufacturing process is defined during this stage based on knowledge gained through. Since 2011 the fda recommends a lifecycle approach to process validation, including. Manufacturing Process Qualification.
From www.slideteam.net
Process Flow Chart For Manufacturing Company Powerpoint Ppt Template Manufacturing Process Qualification Process validation is a critical part of quality assurance in the manufacturing industry. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. It involves the collection and analysis of data to ensure that a process consistently produces.. Manufacturing Process Qualification.
From www.slideteam.net
Manufacturing Quality Control Flow Chart Diagram PPT Sample Manufacturing Process Qualification The commercial manufacturing process is defined during this stage based on knowledge gained through. A who guide to good manufacturing practice (gmp) requirements. It involves the collection and analysis of data to ensure that a process consistently produces. Its purpose is to ensure ongoing product quality. Process validation is a critical part of quality assurance in the manufacturing industry. Establishing. Manufacturing Process Qualification.
From www.researchgate.net
A broad representation of AM qualification flow about the material Manufacturing Process Qualification The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. Process validation is a critical part of quality assurance in the manufacturing industry. Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. Its purpose is to ensure ongoing product quality. It involves the collection and analysis of. Manufacturing Process Qualification.
From www.dozuki.com
5 Ways To Improve Quality Control in Manufacturing Manufacturing Process Qualification Its purpose is to ensure ongoing product quality. It involves the collection and analysis of data to ensure that a process consistently produces. Process validation is a critical part of quality assurance in the manufacturing industry. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. The commercial manufacturing process is defined during this stage. Manufacturing Process Qualification.
From data.epo.org
MANUFACTURING PROCESS QUALIFICATION SYSTEM AND METHOD Patent 4261637 Manufacturing Process Qualification Since 2011 the fda recommends a lifecycle approach to process validation, including three. Process validation is a critical part of quality assurance in the manufacturing industry. It involves the collection and analysis of data to ensure that a process consistently produces. The commercial manufacturing process is defined during this stage based on knowledge gained through. Validate the process to ensure. Manufacturing Process Qualification.
From www.slideserve.com
PPT Process Validation General Principles and Practices PowerPoint Manufacturing Process Qualification Process validation is a critical part of quality assurance in the manufacturing industry. Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. It involves the collection and analysis of data to ensure that a process consistently produces. Validate the process to ensure that the process consistently deliver quality products. Since 2011 the. Manufacturing Process Qualification.
From techqualitypedia.com
Manufacturing Processes Types Manufacturing Processes List Manufacturing Process Qualification A who guide to good manufacturing practice (gmp) requirements. The commercial manufacturing process is defined during this stage based on knowledge gained through. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. Process validation is a critical part of quality assurance in the manufacturing industry. It involves the collection and analysis of data to. Manufacturing Process Qualification.
From www.getreskilled.com
Qualification vs Validation in Pharma GetReskilled Manufacturing Process Qualification Since 2011 the fda recommends a lifecycle approach to process validation, including three. It involves the collection and analysis of data to ensure that a process consistently produces. Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification.. Manufacturing Process Qualification.
From www.hwmfg.com
Our Quality Assurance Process at H&W Manufacturing H&W Manufacturing Manufacturing Process Qualification Process validation is a critical part of quality assurance in the manufacturing industry. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. A who guide to good manufacturing practice (gmp) requirements. It involves the collection and analysis of data to ensure that a process consistently produces. Its purpose is to ensure ongoing product quality.. Manufacturing Process Qualification.
From www.pharmaqualification.com
Prospective Process Validation Manufacturing Process Qualification The commercial manufacturing process is defined during this stage based on knowledge gained through. Validate the process to ensure that the process consistently deliver quality products. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. It involves the collection and analysis of data to ensure that a process consistently produces. Since 2011 the fda. Manufacturing Process Qualification.
From dokumen.tips
(PDF) Manufacturing Process Qualification & Manufacturing Process Qualification Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. A who guide to good manufacturing practice (gmp) requirements. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. The commercial manufacturing process is defined during this stage based on knowledge gained through. Since 2011 the fda recommends. Manufacturing Process Qualification.
From www.slideserve.com
PPT Eisai Production Operations Employee Development PowerPoint Manufacturing Process Qualification Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. The commercial manufacturing process is defined during this stage based on knowledge gained through. Its purpose is to ensure ongoing product quality. Since 2011 the fda recommends a lifecycle approach to process validation, including three. Process validation is a critical part of quality. Manufacturing Process Qualification.
From www.pinterest.dk
This image shows the summary of the Production Part Approval Process Manufacturing Process Qualification Its purpose is to ensure ongoing product quality. It involves the collection and analysis of data to ensure that a process consistently produces. Validate the process to ensure that the process consistently deliver quality products. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. The commercial manufacturing process is defined during this stage based. Manufacturing Process Qualification.
From www.slideshare.net
AdditiveManufacturingQualificationCertificationandImplementation Manufacturing Process Qualification It involves the collection and analysis of data to ensure that a process consistently produces. Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. Its purpose is to ensure ongoing product quality. Process validation is a critical. Manufacturing Process Qualification.
From kvalito.ch
How is a system validated? Kvalito Manufacturing Process Qualification Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. The commercial manufacturing process is defined during this stage based on knowledge gained through. Its purpose is to ensure ongoing product quality. It involves the collection and analysis. Manufacturing Process Qualification.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and Manufacturing Process Qualification A who guide to good manufacturing practice (gmp) requirements. It involves the collection and analysis of data to ensure that a process consistently produces. Its purpose is to ensure ongoing product quality. Since 2011 the fda recommends a lifecycle approach to process validation, including three. Process validation is a critical part of quality assurance in the manufacturing industry. The process. Manufacturing Process Qualification.
From www.jasonmolding.com
FIVE BASIC REQUIREMENTS OF PLASTIC INJECTION MOLDING MANUFACTURING Manufacturing Process Qualification A who guide to good manufacturing practice (gmp) requirements. Validate the process to ensure that the process consistently deliver quality products. It involves the collection and analysis of data to ensure that a process consistently produces. Its purpose is to ensure ongoing product quality. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. Process. Manufacturing Process Qualification.
From www.barnesglobaladvisors.com
The Barnes Global Advisors Additive Manufacturing Consulting Manufacturing Process Qualification Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. Since 2011 the fda recommends a lifecycle approach to process validation, including three. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. Process validation is a critical part of quality assurance in the manufacturing industry. The commercial. Manufacturing Process Qualification.
From www.researchgate.net
(PDF) Qualification Challenges with Additive Manufacturing in Space Manufacturing Process Qualification Since 2011 the fda recommends a lifecycle approach to process validation, including three. It involves the collection and analysis of data to ensure that a process consistently produces. Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. Process validation is a critical part of quality assurance in the manufacturing industry. The process. Manufacturing Process Qualification.
From www.presentationeze.com
Equipment Validation Facility Qualification Material Manufacturing Process Qualification Validate the process to ensure that the process consistently deliver quality products. Its purpose is to ensure ongoing product quality. The commercial manufacturing process is defined during this stage based on knowledge gained through. A who guide to good manufacturing practice (gmp) requirements. It involves the collection and analysis of data to ensure that a process consistently produces. The process. Manufacturing Process Qualification.
From www.slideserve.com
PPT Process Validation General Principles and Practices PowerPoint Manufacturing Process Qualification Its purpose is to ensure ongoing product quality. It involves the collection and analysis of data to ensure that a process consistently produces. The commercial manufacturing process is defined during this stage based on knowledge gained through. Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. Process validation is a critical part. Manufacturing Process Qualification.
From www.researchgate.net
The process of qualification and certification with the steps of raw Manufacturing Process Qualification The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. Process validation is a critical part of quality assurance in the manufacturing industry. Validate the process to ensure that the process consistently deliver quality products. It involves the collection and analysis of data to ensure that a process consistently produces. Establishing by key objective evidence. Manufacturing Process Qualification.
From www.researchgate.net
Flowchart summarising the process of qualification/certification of a Manufacturing Process Qualification It involves the collection and analysis of data to ensure that a process consistently produces. Validate the process to ensure that the process consistently deliver quality products. Process validation is a critical part of quality assurance in the manufacturing industry. The commercial manufacturing process is defined during this stage based on knowledge gained through. Its purpose is to ensure ongoing. Manufacturing Process Qualification.
From www.kmcec.com
Manufacturing Process KMC Engineering Company Manufacturing Process Qualification A who guide to good manufacturing practice (gmp) requirements. The commercial manufacturing process is defined during this stage based on knowledge gained through. The process performance qualification (ppq) protocol is a fundamental component of process validation and qualification. Process validation is a critical part of quality assurance in the manufacturing industry. Validate the process to ensure that the process consistently. Manufacturing Process Qualification.
From www.slideserve.com
PPT Qualification And Validation In Pharmaceutical Manufacturing Manufacturing Process Qualification Process validation is a critical part of quality assurance in the manufacturing industry. The commercial manufacturing process is defined during this stage based on knowledge gained through. It involves the collection and analysis of data to ensure that a process consistently produces. Establishing by key objective evidence that all key aspects of the process equipment and ancillary system installation. Validate. Manufacturing Process Qualification.