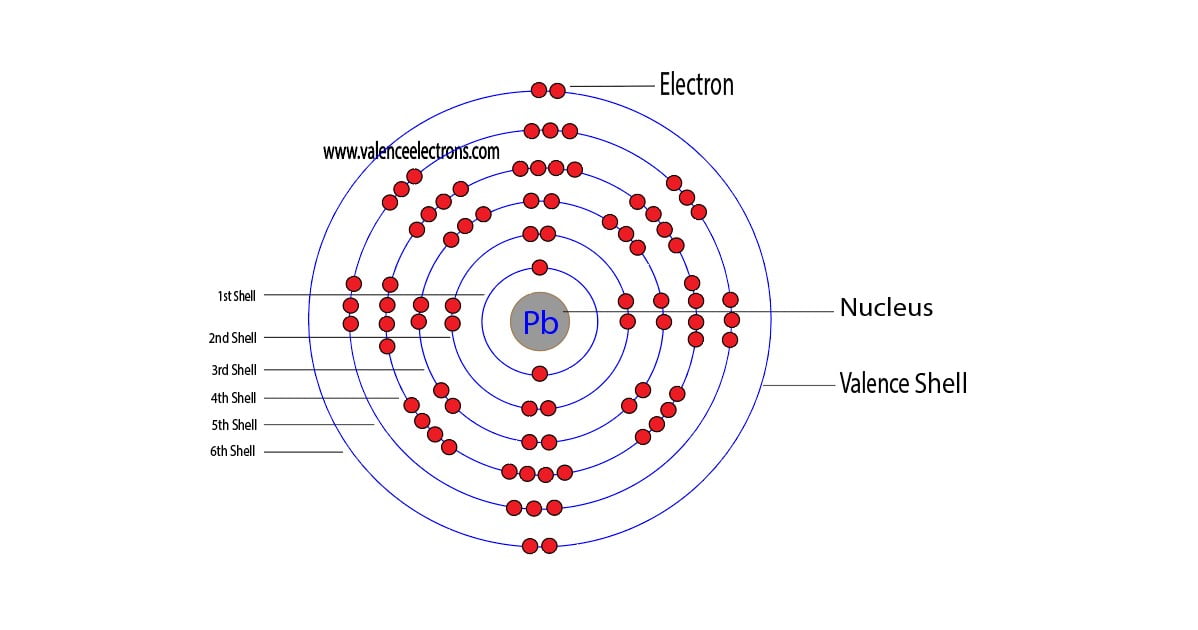
Lead Pb Electron Configuration . Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. This electron configuration shows that the lead ion (pb 4+). The electron configuration of lead is [xe] 4f¹⁴ 5d¹⁰ 6s² 6p². The chemical element present in the periodic table is called lead, its. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. Lead (pb) has an atomic. Electron configuration chart of all elements is mentioned in the table below. The lead electron configuration, represented as 6s2 4f14 5d10 6p2 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2, illustrates the precise Relative atomic mass is also known as atomic weight (symbol:
from valenceelectrons.com
Relative atomic mass is also known as atomic weight (symbol: Electron configuration chart of all elements is mentioned in the table below. Lead (pb) has an atomic. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The chemical element present in the periodic table is called lead, its. The electron configuration of lead is [xe] 4f¹⁴ 5d¹⁰ 6s² 6p². The lead electron configuration, represented as 6s2 4f14 5d10 6p2 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2, illustrates the precise The shorthand electron configuration (or noble gas configuration) as well as. This electron configuration shows that the lead ion (pb 4+).
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+)
Lead Pb Electron Configuration The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. The lead electron configuration, represented as 6s2 4f14 5d10 6p2 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2, illustrates the precise The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. The chemical element present in the periodic table is called lead, its. The shorthand electron configuration (or noble gas configuration) as well as. Relative atomic mass is also known as atomic weight (symbol: Electron configuration chart of all elements is mentioned in the table below. The electron configuration of lead is [xe] 4f¹⁴ 5d¹⁰ 6s² 6p². Lead (pb) has an atomic. Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. This electron configuration shows that the lead ion (pb 4+).
From stock.adobe.com
Lead Pb Post transition metal Chemical Element vector illustration Lead Pb Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. Lead (pb) has an atomic. Electron configuration chart of all elements is mentioned in the table below. The lead electron configuration, represented as 6s2 4f14 5d10 6p2 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2, illustrates the precise Lead is a. Lead Pb Electron Configuration.
From chem.libretexts.org
2.4 Electron Configurations Chemistry LibreTexts Lead Pb Electron Configuration Relative atomic mass is also known as atomic weight (symbol: The electron configuration of lead is [xe] 4f¹⁴ 5d¹⁰ 6s² 6p². Lead (pb) has an atomic. The lead electron configuration, represented as 6s2 4f14 5d10 6p2 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2, illustrates the precise Electron configuration chart of all. Lead Pb Electron Configuration.
From www.alamy.com
Lead (Pb). Diagram of the nuclear composition, electron configuration Lead Pb Electron Configuration Electron configuration chart of all elements is mentioned in the table below. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. This electron configuration shows that the lead ion (pb 4+). Lead (pb) has an atomic. The electron configuration of lead is [xe] 4f¹⁴ 5d¹⁰ 6s² 6p². The electron configuration. Lead Pb Electron Configuration.
From www.schoolmykids.com
Lead (Pb) Element Information, Facts, Properties, Uses Periodic Lead Pb Electron Configuration Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. Electron configuration chart of all elements is mentioned in the table below. Lead (pb) has an atomic. The shorthand electron configuration (or noble gas configuration) as well as. The chemical element present in the periodic table is called lead,. Lead Pb Electron Configuration.
From www.alamy.com
Pb Lead Chemical Element Periodic Table. Single vector illustration Lead Pb Electron Configuration Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The shorthand electron configuration (or noble gas configuration) as well as. Lead (pb) has an atomic. The lead electron configuration, represented as 6s2 4f14 5d10 6p2 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2. Lead Pb Electron Configuration.
From www.youtube.com
Electron Configuration for Pb, Pb2+, and Pb4+ (Lead and Lead Ions Lead Pb Electron Configuration The chemical element present in the periodic table is called lead, its. Relative atomic mass is also known as atomic weight (symbol: The shorthand electron configuration (or noble gas configuration) as well as. This electron configuration shows that the lead ion (pb 4+). Lead is a chemical element with atomic number 82 which means there are 82 protons and 82. Lead Pb Electron Configuration.
From www.nuclear-power.com
Lead Atomic Number Atomic Mass Density of Lead Lead Pb Electron Configuration Relative atomic mass is also known as atomic weight (symbol: The chemical element present in the periodic table is called lead, its. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. Lead is a. Lead Pb Electron Configuration.
From lefteris-kaliambos.fandom.com
EXPLANATION OF LEAD IONIZATIONS Lefteris Kaliambos Wiki Fandom Lead Pb Electron Configuration The chemical element present in the periodic table is called lead, its. Lead (pb) has an atomic. Relative atomic mass is also known as atomic weight (symbol: The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6. Lead Pb Electron Configuration.
From www.slideserve.com
PPT Unit 3 Atomic Structure PowerPoint Presentation, free download Lead Pb Electron Configuration The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. The shorthand electron configuration (or noble. Lead Pb Electron Configuration.
From dreamstime.com
Diagram Representation Of The Element Lead Stock Vector Image 59012885 Lead Pb Electron Configuration The electron configuration of lead is [xe] 4f¹⁴ 5d¹⁰ 6s² 6p². This electron configuration shows that the lead ion (pb 4+). Relative atomic mass is also known as atomic weight (symbol: The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14. Lead Pb Electron Configuration.
From www.alamy.com
Pb Lead, Periodic Table of the Elements, Shell Structure of Lead Lead Pb Electron Configuration Electron configuration chart of all elements is mentioned in the table below. The electron configuration of lead is [xe] 4f¹⁴ 5d¹⁰ 6s² 6p². Lead (pb) has an atomic. This electron configuration shows that the lead ion (pb 4+). The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. The chemical element. Lead Pb Electron Configuration.
From www.chem.fsu.edu
Electron Configurations Lead Pb Electron Configuration The electron configuration of lead is [xe] 4f¹⁴ 5d¹⁰ 6s² 6p². The shorthand electron configuration (or noble gas configuration) as well as. This electron configuration shows that the lead ion (pb 4+). The chemical element present in the periodic table is called lead, its. Lead (pb) has an atomic. Relative atomic mass is also known as atomic weight (symbol: The. Lead Pb Electron Configuration.
From www.istockphoto.com
10+ Electron Configuration Lead Stock Photos, Pictures & RoyaltyFree Lead Pb Electron Configuration The electron configuration of lead is [xe] 4f¹⁴ 5d¹⁰ 6s² 6p². This electron configuration shows that the lead ion (pb 4+). Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The chemical element present in the periodic table is called lead, its. The electron configuration of lead ion. Lead Pb Electron Configuration.
From www.alamy.com
Atom symbol and electron of lead illustration Stock Vector Image & Art Lead Pb Electron Configuration Lead (pb) has an atomic. The chemical element present in the periodic table is called lead, its. Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. The electron configuration. Lead Pb Electron Configuration.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Lead Pb Electron Configuration The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. Electron configuration chart of all elements is mentioned in the table below. Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The electron configuration of lead ion (pb 4+). Lead Pb Electron Configuration.
From www.schoolmykids.com
Lead (Pb) Element Information, Facts, Properties, Uses Periodic Lead Pb Electron Configuration This electron configuration shows that the lead ion (pb 4+). The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. The electron configuration of. Lead Pb Electron Configuration.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Lead Pb Electron Configuration Lead (pb) has an atomic. This electron configuration shows that the lead ion (pb 4+). The shorthand electron configuration (or noble gas configuration) as well as. Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The lead electron configuration, represented as 6s2 4f14 5d10 6p2 or 1s2 2s2. Lead Pb Electron Configuration.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Lead Pb Electron Configuration Lead (pb) has an atomic. The lead electron configuration, represented as 6s2 4f14 5d10 6p2 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2, illustrates the precise This electron configuration shows that the lead ion (pb 4+). The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all. Lead Pb Electron Configuration.
From valenceelectrons.com
Lead(Pb) Electron Configuration and Orbital Diagram Lead Pb Electron Configuration This electron configuration shows that the lead ion (pb 4+). Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its. Lead Pb Electron Configuration.
From chemistryvillage.com
Explanation Lead ion (Pb2+, Pb4+) Electron Configuration Lead Pb Electron Configuration This electron configuration shows that the lead ion (pb 4+). The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. The electron configuration of. Lead Pb Electron Configuration.
From valenceelectrons.com
Lead(Pb) electron configuration and orbital diagram Lead Pb Electron Configuration Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The chemical element present in the periodic table is called lead, its. Lead (pb) has an atomic. The electron configuration of lead is [xe] 4f¹⁴ 5d¹⁰ 6s² 6p². Electron configuration chart of all elements is mentioned in the table. Lead Pb Electron Configuration.
From www.schoolmykids.com
Lead (Pb) Element Information, Facts, Properties, Uses Periodic Lead Pb Electron Configuration Electron configuration chart of all elements is mentioned in the table below. Relative atomic mass is also known as atomic weight (symbol: The lead electron configuration, represented as 6s2 4f14 5d10 6p2 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2, illustrates the precise The shorthand electron configuration (or noble gas configuration) as. Lead Pb Electron Configuration.
From www.britannica.com
Lead Definition, Uses, Properties, & Facts Britannica Lead Pb Electron Configuration Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. This electron configuration shows that the lead ion (pb 4+). The electron configuration of lead is [xe] 4f¹⁴ 5d¹⁰ 6s² 6p². Lead is a chemical element with atomic number 82 which means there are 82 protons and. Lead Pb Electron Configuration.
From www.youtube.com
Electron Configuration of Lead Pb Lesson YouTube Lead Pb Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. Relative atomic mass is also known as atomic weight (symbol: Lead (pb) has an atomic. Electron configuration chart of all elements is mentioned in the table below. The. Lead Pb Electron Configuration.
From stock.adobe.com
Lead Pb Post transition metal Chemical Element vector illustration Lead Pb Electron Configuration Lead (pb) has an atomic. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. The lead electron configuration, represented as 6s2 4f14 5d10 6p2 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2, illustrates the precise This electron configuration shows that the lead. Lead Pb Electron Configuration.
From scienceinfo.com
Lead (Pb) Element Properties, Reactions, Uses Lead Pb Electron Configuration The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. Electron configuration chart of all elements is mentioned in the table below. The electron configuration of lead is [xe] 4f¹⁴ 5d¹⁰ 6s² 6p². This electron. Lead Pb Electron Configuration.
From www.alamy.com
Lead (Pb). Diagram of the nuclear composition and electron Lead Pb Electron Configuration The lead electron configuration, represented as 6s2 4f14 5d10 6p2 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2, illustrates the precise The shorthand electron configuration (or noble gas configuration) as well as. This electron configuration shows that the lead ion (pb 4+). The electron configuration of lead (pb) is described by its. Lead Pb Electron Configuration.
From cabinet.matttroy.net
Lead Periodic Table Electrons Matttroy Lead Pb Electron Configuration The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. The lead electron configuration, represented as 6s2 4f14 5d10 6p2 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10. Lead Pb Electron Configuration.
From iperiodictable.com
How To Find an Valence Lead Electron Configuration (Pb) Lead Pb Electron Configuration Relative atomic mass is also known as atomic weight (symbol: This electron configuration shows that the lead ion (pb 4+). Lead (pb) has an atomic. Electron configuration chart of all elements is mentioned in the table below. The lead electron configuration, represented as 6s2 4f14 5d10 6p2 or 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2. Lead Pb Electron Configuration.
From keplarllp.com
😍 Electron configuration examples. Abbreviated Electron configurations Lead Pb Electron Configuration This electron configuration shows that the lead ion (pb 4+). The chemical element present in the periodic table is called lead, its. Relative atomic mass is also known as atomic weight (symbol: The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f. Lead Pb Electron Configuration.
From www.youtube.com
How to find Protons & Electrons for the Pb2+ and Pb4+ (Lead II and Lead Lead Pb Electron Configuration The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. This electron configuration shows. Lead Pb Electron Configuration.
From www.webelements.com
Elements Periodic Table » Lead » properties of free atoms Lead Pb Electron Configuration Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 4f 14 5s 2 5p 6 5d 10. The shorthand electron configuration. Lead Pb Electron Configuration.
From www.chemistry-online.com
Lead Chemistry Online Lead Pb Electron Configuration This electron configuration shows that the lead ion (pb 4+). Electron configuration chart of all elements is mentioned in the table below. The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. The chemical element present in the periodic table is called lead, its. The lead electron configuration, represented as 6s2. Lead Pb Electron Configuration.
From material-properties.org
Lead Protons Neutrons Electrons Electron Configuration Lead Pb Electron Configuration This electron configuration shows that the lead ion (pb 4+). The electron configuration of lead (pb) is described by its atomic structure, having 82 electrons distributed among its energy. The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of lead ion (pb 4+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d. Lead Pb Electron Configuration.
From www.webelements.com
Elements Periodic Table » Lead » properties of free atoms Lead Pb Electron Configuration This electron configuration shows that the lead ion (pb 4+). Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic. Relative atomic mass is also known as atomic weight (symbol: The shorthand electron configuration (or noble gas configuration) as well as. The electron configuration of lead (pb) is described. Lead Pb Electron Configuration.