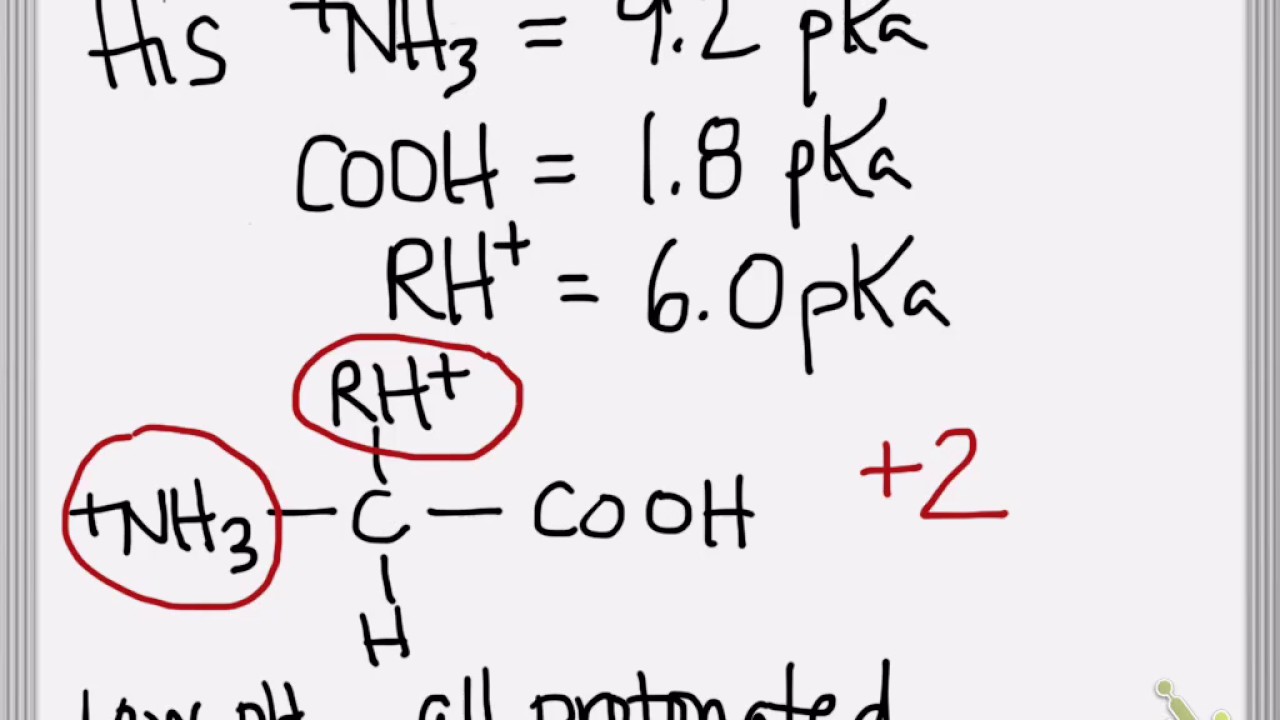
How To Calculate Pi Of Amino Acid Chain . Prot pi [prɑːt paɪ] | protein tool. It can be calculated by the average of the relevant. Raise the ph above the highest pka value and. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. To calculate the isoelectric point, you need to find the ph at which the molecule possesses a net charge of zero. Select a ph between the second and the third pka value of the amino acid and determine the net charge of the amino acid; Isoelectric point of an amino acid is the $\mathrm{ph}$ at which the molecule carries no net charge [1]. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. It can be easily calculated using the formula, pi = pka1 + pka2/ 2 for molecules with two ionizable groups, while for molecules with more than two ionizable groups, pi is the. The zwitterion of an amino acid exists at a ph. Thus, the pi for alanine is calculated to be: Prot pi | protein tool calculates isoelectric point and net charge of proteins, as well as the exact mass and the. This means that the positive.
from www.youtube.com
For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. This means that the positive. It can be easily calculated using the formula, pi = pka1 + pka2/ 2 for molecules with two ionizable groups, while for molecules with more than two ionizable groups, pi is the. Raise the ph above the highest pka value and. To calculate the isoelectric point, you need to find the ph at which the molecule possesses a net charge of zero. Thus, the pi for alanine is calculated to be: The zwitterion of an amino acid exists at a ph. It can be calculated by the average of the relevant. Prot pi [prɑːt paɪ] | protein tool. Prot pi | protein tool calculates isoelectric point and net charge of proteins, as well as the exact mass and the.
Calculating the pI of an amino acid YouTube
How To Calculate Pi Of Amino Acid Chain It can be calculated by the average of the relevant. Prot pi | protein tool calculates isoelectric point and net charge of proteins, as well as the exact mass and the. To calculate the isoelectric point, you need to find the ph at which the molecule possesses a net charge of zero. Select a ph between the second and the third pka value of the amino acid and determine the net charge of the amino acid; When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. Isoelectric point of an amino acid is the $\mathrm{ph}$ at which the molecule carries no net charge [1]. Thus, the pi for alanine is calculated to be: Raise the ph above the highest pka value and. This means that the positive. Prot pi [prɑːt paɪ] | protein tool. It can be easily calculated using the formula, pi = pka1 + pka2/ 2 for molecules with two ionizable groups, while for molecules with more than two ionizable groups, pi is the. The zwitterion of an amino acid exists at a ph. It can be calculated by the average of the relevant. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups.
From www.youtube.com
How to calculate Isoelectric point (pI) of Amino acids YouTube How To Calculate Pi Of Amino Acid Chain For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. To calculate the isoelectric point, you need to find the ph at which the molecule possesses a net charge of zero. Select a ph between the second and the third pka value of the amino. How To Calculate Pi Of Amino Acid Chain.
From www.youtube.com
The Charge and PI of Amino Acid (Tyrosine) Explained Chemistry YouTube How To Calculate Pi Of Amino Acid Chain When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. Select a ph between the second and the third pka value of the amino acid and determine the net charge of the amino acid; For simple amino acids such as alanine, the pi is. How To Calculate Pi Of Amino Acid Chain.
From cwsimons.com
How to Determine the Net Charge of Amino Acids Food Science Toolbox How To Calculate Pi Of Amino Acid Chain For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. Prot pi [prɑːt paɪ] | protein tool. It can be easily calculated using the formula, pi = pka1 + pka2/ 2 for molecules with two ionizable groups, while for molecules with more than two ionizable. How To Calculate Pi Of Amino Acid Chain.
From foodsciencetoolbox.com
How to Calculate PI of Amino Acid Food Science Toolbox How To Calculate Pi Of Amino Acid Chain Thus, the pi for alanine is calculated to be: Prot pi [prɑːt paɪ] | protein tool. It can be easily calculated using the formula, pi = pka1 + pka2/ 2 for molecules with two ionizable groups, while for molecules with more than two ionizable groups, pi is the. For simple amino acids such as alanine, the pi is an average. How To Calculate Pi Of Amino Acid Chain.
From cwsimons.com
How to Determine the Isoelectric Point (pI) of Amino Acids With and How To Calculate Pi Of Amino Acid Chain It can be calculated by the average of the relevant. This means that the positive. Isoelectric point of an amino acid is the $\mathrm{ph}$ at which the molecule carries no net charge [1]. Select a ph between the second and the third pka value of the amino acid and determine the net charge of the amino acid; The zwitterion of. How To Calculate Pi Of Amino Acid Chain.
From www.youtube.com
Calculating the pI of an Amino Acid YouTube How To Calculate Pi Of Amino Acid Chain For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. Thus, the pi for alanine is calculated to be: It can be easily calculated using the formula, pi = pka1 + pka2/ 2 for molecules with two ionizable groups, while for molecules with more than. How To Calculate Pi Of Amino Acid Chain.
From peptideweb.com
Amino acid properties How To Calculate Pi Of Amino Acid Chain Select a ph between the second and the third pka value of the amino acid and determine the net charge of the amino acid; When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. For simple amino acids such as alanine, the pi is. How To Calculate Pi Of Amino Acid Chain.
From lokinp.weebly.com
Calculate pi of amino acid with r group lokinp How To Calculate Pi Of Amino Acid Chain When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. It can be calculated by the average of the relevant. Isoelectric point of an amino acid is the $\mathrm{ph}$ at which the molecule carries no net charge [1]. Prot pi | protein tool calculates. How To Calculate Pi Of Amino Acid Chain.
From leah4sci.com
Isoelectric Point of Amino Acids Tutorial video with 3 pka shortcut How To Calculate Pi Of Amino Acid Chain This means that the positive. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. It can be easily calculated using the formula, pi = pka1 + pka2/ 2 for molecules with two ionizable groups, while for molecules with more than two ionizable groups, pi. How To Calculate Pi Of Amino Acid Chain.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master How To Calculate Pi Of Amino Acid Chain It can be calculated by the average of the relevant. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. Thus, the pi for alanine is calculated to be: To calculate the isoelectric point, you need to find the ph at which the molecule possesses. How To Calculate Pi Of Amino Acid Chain.
From www.reddit.com
calculating pI for acidic/basic amino acids? r/Mcat How To Calculate Pi Of Amino Acid Chain Thus, the pi for alanine is calculated to be: It can be calculated by the average of the relevant. It can be easily calculated using the formula, pi = pka1 + pka2/ 2 for molecules with two ionizable groups, while for molecules with more than two ionizable groups, pi is the. Isoelectric point of an amino acid is the $\mathrm{ph}$. How To Calculate Pi Of Amino Acid Chain.
From www.youtube.com
How to calculate isoelectric point of amino acid? Calculating the pI How To Calculate Pi Of Amino Acid Chain The zwitterion of an amino acid exists at a ph. Isoelectric point of an amino acid is the $\mathrm{ph}$ at which the molecule carries no net charge [1]. Prot pi | protein tool calculates isoelectric point and net charge of proteins, as well as the exact mass and the. It can be easily calculated using the formula, pi = pka1. How To Calculate Pi Of Amino Acid Chain.
From leah4sci.com
Peptide Charge and Isoelectric Point Shortcut MCAT and Organic How To Calculate Pi Of Amino Acid Chain To calculate the isoelectric point, you need to find the ph at which the molecule possesses a net charge of zero. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. Raise the ph above the highest pka value and. It can be calculated. How To Calculate Pi Of Amino Acid Chain.
From www.youtube.com
Calculating the pI of an amino acid YouTube How To Calculate Pi Of Amino Acid Chain Prot pi | protein tool calculates isoelectric point and net charge of proteins, as well as the exact mass and the. This means that the positive. Raise the ph above the highest pka value and. Prot pi [prɑːt paɪ] | protein tool. It can be calculated by the average of the relevant. The zwitterion of an amino acid exists at. How To Calculate Pi Of Amino Acid Chain.
From cwsimons.com
How to Determine Isoelectric Point (pI) of Peptides Food Science Toolbox How To Calculate Pi Of Amino Acid Chain This means that the positive. To calculate the isoelectric point, you need to find the ph at which the molecule possesses a net charge of zero. Select a ph between the second and the third pka value of the amino acid and determine the net charge of the amino acid; Raise the ph above the highest pka value and. Thus,. How To Calculate Pi Of Amino Acid Chain.
From www.youtube.com
How to find a number of Amino acids in protein chain? YouTube How To Calculate Pi Of Amino Acid Chain This means that the positive. The zwitterion of an amino acid exists at a ph. Raise the ph above the highest pka value and. It can be easily calculated using the formula, pi = pka1 + pka2/ 2 for molecules with two ionizable groups, while for molecules with more than two ionizable groups, pi is the. To calculate the isoelectric. How To Calculate Pi Of Amino Acid Chain.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master How To Calculate Pi Of Amino Acid Chain To calculate the isoelectric point, you need to find the ph at which the molecule possesses a net charge of zero. It can be calculated by the average of the relevant. Select a ph between the second and the third pka value of the amino acid and determine the net charge of the amino acid; It can be easily calculated. How To Calculate Pi Of Amino Acid Chain.
From dikipanda.weebly.com
Calculate pi of amino acid dikipanda How To Calculate Pi Of Amino Acid Chain Isoelectric point of an amino acid is the $\mathrm{ph}$ at which the molecule carries no net charge [1]. To calculate the isoelectric point, you need to find the ph at which the molecule possesses a net charge of zero. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34). How To Calculate Pi Of Amino Acid Chain.
From www.youtube.com
How to find pi of amino acid? YouTube How To Calculate Pi Of Amino Acid Chain The zwitterion of an amino acid exists at a ph. Select a ph between the second and the third pka value of the amino acid and determine the net charge of the amino acid; For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. Prot. How To Calculate Pi Of Amino Acid Chain.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master How To Calculate Pi Of Amino Acid Chain For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. To calculate the isoelectric point, you need to find the ph at which the molecule possesses a net charge of zero. Prot pi | protein tool calculates isoelectric point and net charge of proteins, as. How To Calculate Pi Of Amino Acid Chain.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master How To Calculate Pi Of Amino Acid Chain Prot pi | protein tool calculates isoelectric point and net charge of proteins, as well as the exact mass and the. It can be calculated by the average of the relevant. Thus, the pi for alanine is calculated to be: This means that the positive. The zwitterion of an amino acid exists at a ph. Raise the ph above the. How To Calculate Pi Of Amino Acid Chain.
From gbee.edu.vn
Isoelectric Points of Amino Acids (and How To Calculate Them) Gbee How To Calculate Pi Of Amino Acid Chain Thus, the pi for alanine is calculated to be: When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. The zwitterion of an amino acid exists at a ph. It can be easily calculated using the formula, pi = pka1 + pka2/ 2 for. How To Calculate Pi Of Amino Acid Chain.
From gacsach.vn
Isoelectric Points Of Amino Acids (and How To Calculate, 57 OFF How To Calculate Pi Of Amino Acid Chain Prot pi | protein tool calculates isoelectric point and net charge of proteins, as well as the exact mass and the. To calculate the isoelectric point, you need to find the ph at which the molecule possesses a net charge of zero. Prot pi [prɑːt paɪ] | protein tool. For simple amino acids such as alanine, the pi is an. How To Calculate Pi Of Amino Acid Chain.
From cwsimons.com
How to Determine the Isoelectric Point (pI) of Amino Acids With and How To Calculate Pi Of Amino Acid Chain This means that the positive. To calculate the isoelectric point, you need to find the ph at which the molecule possesses a net charge of zero. Isoelectric point of an amino acid is the $\mathrm{ph}$ at which the molecule carries no net charge [1]. Select a ph between the second and the third pka value of the amino acid and. How To Calculate Pi Of Amino Acid Chain.
From wafiyanire.blogspot.com
32+ How To Calculate Pi Of Amino Acids WafiYanire How To Calculate Pi Of Amino Acid Chain When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. Select a ph between the second and the third pka value of the amino acid and determine the net charge of the amino acid; Prot pi | protein tool calculates isoelectric point and net. How To Calculate Pi Of Amino Acid Chain.
From www.slideserve.com
PPT Amino acids PowerPoint Presentation, free download ID3960157 How To Calculate Pi Of Amino Acid Chain To calculate the isoelectric point, you need to find the ph at which the molecule possesses a net charge of zero. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. Thus, the pi for alanine is calculated to be: For simple amino acids. How To Calculate Pi Of Amino Acid Chain.
From chem.libretexts.org
13.1 Amino Acids Chemistry LibreTexts How To Calculate Pi Of Amino Acid Chain To calculate the isoelectric point, you need to find the ph at which the molecule possesses a net charge of zero. Prot pi | protein tool calculates isoelectric point and net charge of proteins, as well as the exact mass and the. Prot pi [prɑːt paɪ] | protein tool. For simple amino acids such as alanine, the pi is an. How To Calculate Pi Of Amino Acid Chain.
From reecehayes.z13.web.core.windows.net
Amino Acid Chain Chart How To Calculate Pi Of Amino Acid Chain Select a ph between the second and the third pka value of the amino acid and determine the net charge of the amino acid; Prot pi [prɑːt paɪ] | protein tool. Raise the ph above the highest pka value and. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion. How To Calculate Pi Of Amino Acid Chain.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master How To Calculate Pi Of Amino Acid Chain Select a ph between the second and the third pka value of the amino acid and determine the net charge of the amino acid; Isoelectric point of an amino acid is the $\mathrm{ph}$ at which the molecule carries no net charge [1]. This means that the positive. Prot pi [prɑːt paɪ] | protein tool. For simple amino acids such as. How To Calculate Pi Of Amino Acid Chain.
From www.chemzipper.com
to Chem What is the Isoelectric points of How To Calculate Pi Of Amino Acid Chain This means that the positive. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. For simple amino acids such as alanine, the pi is an average of the pk a 's of the carboxyl (2.34) and ammonium (9.69) groups. Prot pi | protein. How To Calculate Pi Of Amino Acid Chain.
From spicystart.weebly.com
Calculate pi of amino acid in the middle of a polypeptide spicystart How To Calculate Pi Of Amino Acid Chain To calculate the isoelectric point, you need to find the ph at which the molecule possesses a net charge of zero. Raise the ph above the highest pka value and. Select a ph between the second and the third pka value of the amino acid and determine the net charge of the amino acid; Prot pi | protein tool calculates. How To Calculate Pi Of Amino Acid Chain.
From lokicoupons.weebly.com
Calculate pi of amino acid chain lokicoupons How To Calculate Pi Of Amino Acid Chain It can be easily calculated using the formula, pi = pka1 + pka2/ 2 for molecules with two ionizable groups, while for molecules with more than two ionizable groups, pi is the. Thus, the pi for alanine is calculated to be: This means that the positive. Prot pi [prɑːt paɪ] | protein tool. It can be calculated by the average. How To Calculate Pi Of Amino Acid Chain.
From ysmyte.weebly.com
Calculate pi of amino acid with r group ysmyte How To Calculate Pi Of Amino Acid Chain When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. Select a ph between the second and the third pka value of the amino acid and determine the net charge of the amino acid; Raise the ph above the highest pka value and. To. How To Calculate Pi Of Amino Acid Chain.
From www.youtube.com
How To Calculate The Isoelectric Point of Amino Acids and Zwitterions How To Calculate Pi Of Amino Acid Chain It can be easily calculated using the formula, pi = pka1 + pka2/ 2 for molecules with two ionizable groups, while for molecules with more than two ionizable groups, pi is the. To calculate the isoelectric point, you need to find the ph at which the molecule possesses a net charge of zero. Isoelectric point of an amino acid is. How To Calculate Pi Of Amino Acid Chain.
From www.masterorganicchemistry.com
Isoelectric Points of Amino Acids (and How To Calculate Them) Master How To Calculate Pi Of Amino Acid Chain To calculate the isoelectric point, you need to find the ph at which the molecule possesses a net charge of zero. When an amino acid contains both a plus and a minus charge in the backbone, it is called a zwitterion and has an overall neutral charge. This means that the positive. For simple amino acids such as alanine, the. How To Calculate Pi Of Amino Acid Chain.