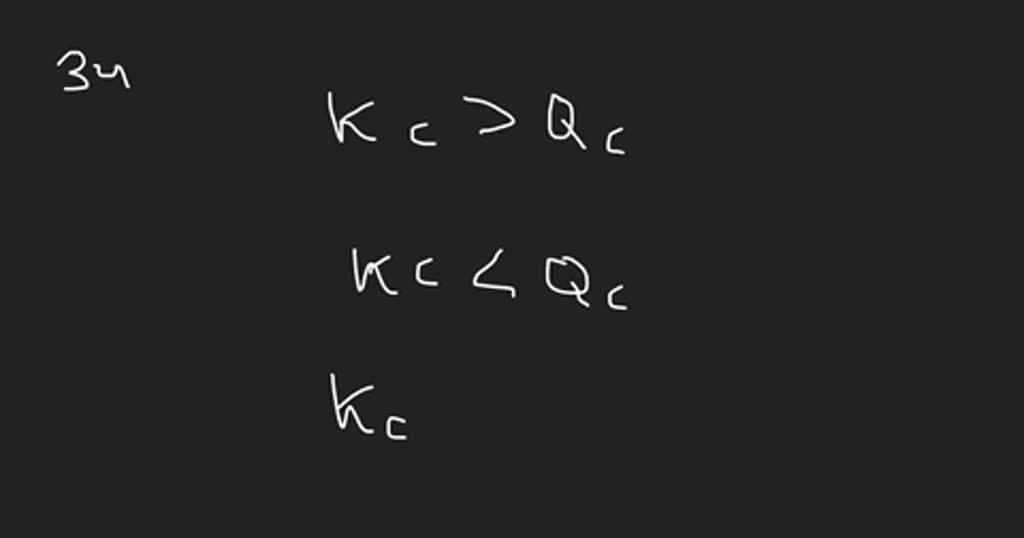Bromine Nitric Oxide . The reaction between nitric oxide and bromine is described by the following chemical equation; Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k. Step by step explanations answered by teachers vaia original! 2no (g) + br2(g) ⇌ 2nobr (g) when. Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. Q97cp nitric oxide and bromine at initial partial pressure. 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: Nitric oxide and bromine were allowed to react in a sealed container.
from www.numerade.com
2no (g) + br2(g) ⇌ 2nobr (g) when. Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. The reaction between nitric oxide and bromine is described by the following chemical equation; 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: Q97cp nitric oxide and bromine at initial partial pressure. Nitric oxide and bromine were allowed to react in a sealed container. Step by step explanations answered by teachers vaia original! Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k.
SOLVEDAt a certain temperature, bromine and nitric oxide react to form
Bromine Nitric Oxide Step by step explanations answered by teachers vaia original! Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. Q97cp nitric oxide and bromine at initial partial pressure. Nitric oxide and bromine were allowed to react in a sealed container. 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: The reaction between nitric oxide and bromine is described by the following chemical equation; Step by step explanations answered by teachers vaia original! 2no (g) + br2(g) ⇌ 2nobr (g) when. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k.
From www.chegg.com
Solved Nitric oxide and bromine at initial partial pressures Bromine Nitric Oxide Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: Step by step explanations answered by teachers vaia original! Nitric oxide and bromine were allowed to react in a sealed container. 2no (g) + br2(g) ⇌ 2nobr (g) when. Q97cp nitric oxide and. Bromine Nitric Oxide.
From www.toppr.com
(g) Match the following. i) Chemical name of dinitrogen oxide ii Bromine Nitric Oxide Q97cp nitric oxide and bromine at initial partial pressure. Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: Step by step explanations answered by teachers vaia original! The reaction between nitric oxide and bromine is described by the following chemical equation; Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr,. Bromine Nitric Oxide.
From www.numerade.com
SOLVEDAt a certain temperature, bromine and nitric oxide react to form Bromine Nitric Oxide The reaction between nitric oxide and bromine is described by the following chemical equation; Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: Step by step explanations answered by teachers vaia original! 2no. Bromine Nitric Oxide.
From www.chegg.com
Solved Suppose the reaction between nitric oxide and bromine Bromine Nitric Oxide The reaction between nitric oxide and bromine is described by the following chemical equation; 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Q97cp nitric oxide and bromine at initial partial pressure. Step by step explanations answered by teachers vaia original! Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react. Bromine Nitric Oxide.
From www.chegg.com
Solved Suppose the reaction between nitric oxide and bromine Bromine Nitric Oxide Step by step explanations answered by teachers vaia original! The reaction between nitric oxide and bromine is described by the following chemical equation; Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: Suppose the reaction. Bromine Nitric Oxide.
From www.chegg.com
Solved Suppose the reaction between nitric oxide and bromine Bromine Nitric Oxide 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k. 2no (g) + br2(g) ⇌ 2nobr (g) when. Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: Q97cp nitric oxide and bromine at initial partial. Bromine Nitric Oxide.
From www.numerade.com
SOLVEDThe following mechanism has been proposed for the reaction Bromine Nitric Oxide 2no (g) + br2(g) ⇌ 2nobr (g) when. Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: Step by step explanations answered by teachers vaia original! Suppose the reaction between nitric oxide and bromine proceeds. Bromine Nitric Oxide.
From www.numerade.com
SOLVEDNitric oxide and bromine were allowed to react in a sealed Bromine Nitric Oxide Step by step explanations answered by teachers vaia original! 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Q97cp nitric oxide and bromine at initial partial pressure. 2no (g) + br2(g) ⇌ 2nobr (g) when. Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: The reaction between nitric oxide and bromine is described by the following. Bromine Nitric Oxide.
From www.chegg.com
Solved Suppose the reaction between nitric oxide and bromine Bromine Nitric Oxide The reaction between nitric oxide and bromine is described by the following chemical equation; Q97cp nitric oxide and bromine at initial partial pressure. Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: Nitric. Bromine Nitric Oxide.
From www.chegg.com
Suppose the reaction between nitric oxide and bromine Bromine Nitric Oxide Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k. Step by step explanations answered by teachers vaia original! 2no)g + br_2 (g) right arrow 2nobr (g) suppose.. Bromine Nitric Oxide.
From www.chegg.com
Solved Bromine gas and nitric oxide can combine to form Bromine Nitric Oxide Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. Step by step explanations answered by teachers vaia original! The reaction between nitric oxide and bromine is described by the following chemical equation; Q97cp nitric oxide and bromine at initial partial pressure. 2no)g + br_2 (g) right arrow 2nobr (g). Bromine Nitric Oxide.
From www.chegg.com
Solved At a certain temperature, bromine and nitric oxide Bromine Nitric Oxide Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: 2no (g) + br2(g) ⇌ 2nobr (g) when. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k. Q97cp nitric oxide. Bromine Nitric Oxide.
From www.chegg.com
Solved Suppose the reaction between nitric oxide and bromine Bromine Nitric Oxide Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. Q97cp nitric oxide and bromine at initial partial pressure. Nitric oxide and bromine were allowed to react in a sealed container. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react. Bromine Nitric Oxide.
From www.numerade.com
SOLVED At a certain temperature, bromine and nitric oxide react to Bromine Nitric Oxide 2no (g) + br2(g) ⇌ 2nobr (g) when. 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Nitric oxide and bromine were allowed to react in a sealed container. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k. Nitric oxide reacts with br2 and gives nitrosyl bromide. Bromine Nitric Oxide.
From www.chegg.com
Solved At a certain temperature, bromine and nitric oxide Bromine Nitric Oxide Step by step explanations answered by teachers vaia original! Nitric oxide and bromine were allowed to react in a sealed container. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k. Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: 2no)g + br_2 (g). Bromine Nitric Oxide.
From www.bartleby.com
Answered Nitric oxide is a highly reactive,… bartleby Bromine Nitric Oxide Nitric oxide and bromine were allowed to react in a sealed container. The reaction between nitric oxide and bromine is described by the following chemical equation; 2no (g) + br2(g) ⇌ 2nobr (g) when. 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: Nitric oxide and bromine. Bromine Nitric Oxide.
From www.chegg.com
Solved Suppose the reaction between nitric oxide and bromine Bromine Nitric Oxide Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: Nitric oxide and bromine were allowed to react in a sealed container. Q97cp nitric oxide and bromine at initial partial pressure. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k. Step by step. Bromine Nitric Oxide.
From www.alamy.com
Chemist atom of Bromine diagram Stock Vector Image & Art Alamy Bromine Nitric Oxide Q97cp nitric oxide and bromine at initial partial pressure. 2no (g) + br2(g) ⇌ 2nobr (g) when. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k. 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Suppose the reaction between nitric oxide and bromine proceeds by the following. Bromine Nitric Oxide.
From www.chegg.com
Solved Suppose the reaction between nitric oxide and bromine Bromine Nitric Oxide 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: Step by step explanations answered by teachers vaia original! The reaction between nitric oxide and bromine is described by the following chemical equation; Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: 2no. Bromine Nitric Oxide.
From www.toppr.com
36. At a certain temperature, bromine and nitric oxide react to form Bromine Nitric Oxide Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. Q97cp nitric oxide and bromine at initial partial pressure. Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: Step by step explanations. Bromine Nitric Oxide.
From www.chegg.com
Solved Suppose the reaction between nitric oxide and bromine Bromine Nitric Oxide Step by step explanations answered by teachers vaia original! Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed. Bromine Nitric Oxide.
From www.numerade.com
SOLVEDThe gas phase reaction of nitric oxide, NO, with bromine, Brz Bromine Nitric Oxide 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: The reaction between nitric oxide and bromine is described by the following chemical equation; Nitric oxide and bromine at initial partial pressures of \(98.4\). Bromine Nitric Oxide.
From www.numerade.com
SOLVEDAt a certain temperature, bromine and nitric oxide react to form Bromine Nitric Oxide 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Nitric oxide and bromine were allowed to react in a sealed container. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k. Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to. Bromine Nitric Oxide.
From www.chegg.com
Solved Nitric oxide reacts with bromine to produce Nitrosyl Bromine Nitric Oxide Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Q97cp nitric oxide and bromine at initial partial pressure. 2no (g) + br2(g) ⇌ 2nobr (g) when. The reaction between nitric oxide and bromine is described by the following chemical equation;. Bromine Nitric Oxide.
From www.numerade.com
SOLVED Nitric oxide and bromine were allowed to react in a sealed Bromine Nitric Oxide Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Step by step explanations answered by teachers vaia original! Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. Nitric oxide and bromine at initial partial pressures of. Bromine Nitric Oxide.
From www.toppr.com
Nitric oxide reacts with bromine and gives nitrosyl bromide as per Bromine Nitric Oxide The reaction between nitric oxide and bromine is described by the following chemical equation; 2no (g) + br2(g) ⇌ 2nobr (g) when. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k. Nitric oxide and bromine were allowed to react in a sealed container. 2no)g + br_2 (g). Bromine Nitric Oxide.
From www.chegg.com
Solved Nitric oxide and bromine at initial partial pressures Bromine Nitric Oxide 2no (g) + br2(g) ⇌ 2nobr (g) when. Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: The reaction between nitric oxide and bromine is described by the following chemical equation; Step by step explanations. Bromine Nitric Oxide.
From www.chegg.com
Solved Consider the gasphase reaction between nitric oxide Bromine Nitric Oxide 2no (g) + br2(g) ⇌ 2nobr (g) when. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k. Q97cp nitric oxide and bromine at initial partial pressure. Suppose the reaction between nitric oxide and bromine proceeds by the following mechanism: Nitric oxide and bromine were allowed to react. Bromine Nitric Oxide.
From www.answersarena.com
[Solved] 1. Nitric oxide is a highly reactive, colourless Bromine Nitric Oxide Step by step explanations answered by teachers vaia original! Q97cp nitric oxide and bromine at initial partial pressure. 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. 2no (g) + br2(g) ⇌ 2nobr (g) when. Nitric oxide and bromine were. Bromine Nitric Oxide.
From www.chegg.com
Solved suppose the reaction between nitric oxide and bromine Bromine Nitric Oxide Nitric oxide and bromine were allowed to react in a sealed container. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k. Step by step explanations answered by teachers vaia original! The reaction between nitric oxide and bromine is described by the following chemical equation; Nitric oxide and. Bromine Nitric Oxide.
From www.chegg.com
Solved Nitric oxide reacts with bromine NO + 1/2 Br2 → NOBI Bromine Nitric Oxide Nitric oxide and bromine were allowed to react in a sealed container. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k. Step by step explanations answered by teachers vaia original! 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Nitric oxide and bromine at initial partial pressures. Bromine Nitric Oxide.
From www.chegg.com
Solved Nitric oxide reacts with bromine at elevated Bromine Nitric Oxide 2no (g) + br2(g) ⇌ 2nobr (g) when. Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k. Q97cp nitric oxide and bromine at initial partial pressure. Nitric. Bromine Nitric Oxide.
From www.chegg.com
Solved 20) Nitric oxide and bromine at initial pressures of Bromine Nitric Oxide 2no (g) + br2(g) ⇌ 2nobr (g) when. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed to react at \(300.\) k. The reaction between nitric oxide and bromine is described by the following chemical equation; Nitric oxide reacts with br2 and gives nitrosyl bromide as per reaction given below: Nitric oxide. Bromine Nitric Oxide.
From www.chegg.com
Solved 20) Nitric oxide and bromine at initial pressures of Bromine Nitric Oxide Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. The reaction between nitric oxide and bromine is described by the following chemical equation; 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Nitric oxide and bromine at initial partial pressures of 98.4 torr and 41.3 torr, respectively, were allowed. Bromine Nitric Oxide.
From www.chegg.com
Solved Suppose the reaction between nitric oxide and bromine Bromine Nitric Oxide 2no)g + br_2 (g) right arrow 2nobr (g) suppose. Nitric oxide and bromine were allowed to react in a sealed container. Q97cp nitric oxide and bromine at initial partial pressure. Nitric oxide and bromine at initial partial pressures of \(98.4\) and \(41.3\) torr, respectively, were allowed to react at \(300. 2no (g) + br2(g) ⇌ 2nobr (g) when. Step by. Bromine Nitric Oxide.