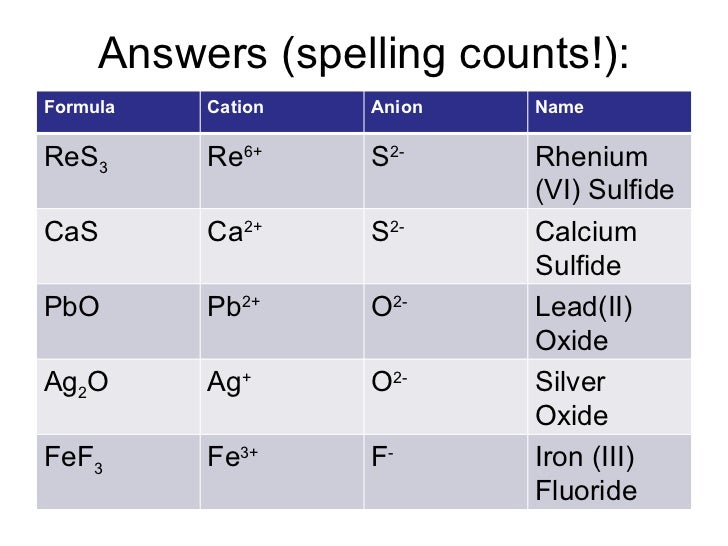
Lead Iv Oxide Cation . Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. The charge of the ion is the number of protons. Write the formula for lead (iv) oxide; It is also called lead dioxide, anhydrous plumbic. Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. There are obviously two types of ions, positive and negative, which are called cation and anion respectively. All the anions are of this type, gaining the number of electrons. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second.
from www.slideshare.net
The charge of the ion is the number of protons. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. There are obviously two types of ions, positive and negative, which are called cation and anion respectively. Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. It is also called lead dioxide, anhydrous plumbic. All the anions are of this type, gaining the number of electrons. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. Write the formula for lead (iv) oxide;
Ionic bonding binary
Lead Iv Oxide Cation The charge of the ion is the number of protons. The charge of the ion is the number of protons. Write the formula for lead (iv) oxide; For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. It is also called lead dioxide, anhydrous plumbic. All the anions are of this type, gaining the number of electrons. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. There are obviously two types of ions, positive and negative, which are called cation and anion respectively.
From slideplayer.com
Chemical Nomenclature Formula Writing & Naming Compounds ppt download Lead Iv Oxide Cation The charge of the ion is the number of protons. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. Pbo 2 is an oxide where the oxidation state of lead is +4 with the. Lead Iv Oxide Cation.
From www.chegg.com
Solved 72. Car batteries use solid lead and lead(IV) oxide Lead Iv Oxide Cation Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. Write the symbol. Lead Iv Oxide Cation.
From www.shutterstock.com
Lead Iv Oxide Properties Chemical Compound Stock Vector (Royalty Free Lead Iv Oxide Cation Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. Write the formula for lead (iv) oxide; Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. Lead (iv) oxide also called. Lead Iv Oxide Cation.
From www.semanticscholar.org
of lead(IV) oxide (PbO2) reductive dissolution role of lead Lead Iv Oxide Cation Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. The charge of the ion is the number of protons. There are. Lead Iv Oxide Cation.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Lead Iv Oxide Cation For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. It is also called lead dioxide, anhydrous plumbic. All the anions are of this type, gaining the number of electrons. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. The charge of the ion is the. Lead Iv Oxide Cation.
From www.alamy.com
Lead (II,IV) oxide in Chemical Watch Glass place next to crystal clear Lead Iv Oxide Cation Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. The charge of the ion is the number of protons. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. There are obviously two types of ions, positive and negative, which are called cation and anion respectively. For each p. Lead Iv Oxide Cation.
From slideplayer.com
Chemistry NOMENCLATURE ppt download Lead Iv Oxide Cation Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. The charge of the ion is the number of protons. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. All the. Lead Iv Oxide Cation.
From www.youtube.com
How to Write the Formula for Lead (IV) nitride YouTube Lead Iv Oxide Cation For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. The charge of the ion is the number of protons. Write the formula for lead (iv) oxide; Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. All the anions are of this type, gaining the number. Lead Iv Oxide Cation.
From www.slideserve.com
PPT Writing and Naming Ionic compounds (criss cross method Lead Iv Oxide Cation Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. There are obviously two types of ions, positive and negative, which are called cation and anion respectively. Write the symbol. Lead Iv Oxide Cation.
From testbook.com
Lead (IV) Oxide Formula Definition, Formula, Production & Uses Lead Iv Oxide Cation Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. There are obviously two types of ions, positive and negative, which are called cation and anion respectively. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. The charge of the ion is the number of protons.. Lead Iv Oxide Cation.
From www.numerade.com
write a balanced equation to represent the of lead(IV Lead Iv Oxide Cation Write the formula for lead (iv) oxide; Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. Pbo. Lead Iv Oxide Cation.
From slideplayer.com
5 Matter and Bonding. ppt download Lead Iv Oxide Cation Write the formula for lead (iv) oxide; Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. The charge of the ion is the number of protons. Lead (iv) oxide also called plumbic oxide, anhydrous. Lead Iv Oxide Cation.
From www.youtube.com
How to Write the Formula for Lead (IV) oxide YouTube Lead Iv Oxide Cation All the anions are of this type, gaining the number of electrons. The charge of the ion is the number of protons. It is also called lead dioxide, anhydrous plumbic. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Write the formula for lead (iv) oxide; There are obviously two types of ions, positive and negative,. Lead Iv Oxide Cation.
From www.youtube.com
Lead `(IV)` oxide is obtained by YouTube Lead Iv Oxide Cation The charge of the ion is the number of protons. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Write the. Lead Iv Oxide Cation.
From www.fishersci.ca
Lead(IV) oxide, ACS, 97.0 min, Thermo Scientific Chemicals Fisher Lead Iv Oxide Cation It is also called lead dioxide, anhydrous plumbic. Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second.. Lead Iv Oxide Cation.
From www.fishersci.se
Lead(II,IV) oxide, red, 98, Thermo Scientific Chemicals Fisher Lead Iv Oxide Cation It is also called lead dioxide, anhydrous plumbic. All the anions are of this type, gaining the number of electrons. Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. Pbo 2 is. Lead Iv Oxide Cation.
From www.alamy.com
Lead (II,IV) oxide in Chemical Watch Glass on white laboratory table Lead Iv Oxide Cation The charge of the ion is the number of protons. Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. It is also called lead dioxide, anhydrous plumbic. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. All the anions are of. Lead Iv Oxide Cation.
From www.numerade.com
SOLVED Important Terms needed for this question. Complete the table Lead Iv Oxide Cation There are obviously two types of ions, positive and negative, which are called cation and anion respectively. It is also called lead dioxide, anhydrous plumbic. Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. The charge of the ion is the number of protons. Write the formula for lead (iv) oxide; Figure. Lead Iv Oxide Cation.
From slideplayer.com
Chemical Reactions & Equations ppt download Lead Iv Oxide Cation Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. All the anions are of this type, gaining the number of electrons. It is also called lead dioxide, anhydrous plumbic. Write the formula for lead (iv) oxide; The charge of the ion is the number of protons. For each p b4+ we need. Lead Iv Oxide Cation.
From www.chegg.com
Solved Lead (IV) oxide dissolves in concentrated Lead Iv Oxide Cation The charge of the ion is the number of protons. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. All the anions are of this type, gaining the number of electrons. Write the formula for lead (iv) oxide; It is also called lead dioxide, anhydrous plumbic. Write the symbol. Lead Iv Oxide Cation.
From www.indiamart.com
Lead (II, IV) Oxide at Rs 910 Battery Oxide in Kolkata ID 15081370697 Lead Iv Oxide Cation The charge of the ion is the number of protons. All the anions are of this type, gaining the number of electrons. Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. Write. Lead Iv Oxide Cation.
From www.coursehero.com
[Solved] What is the lewis ionic structure for Lead (IV) Oxide (PbO2 Lead Iv Oxide Cation Write the formula for lead (iv) oxide; For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. There are obviously two types of ions, positive and negative, which are called. Lead Iv Oxide Cation.
From www.slideserve.com
PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation, free Lead Iv Oxide Cation The charge of the ion is the number of protons. It is also called lead dioxide, anhydrous plumbic. Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. Figure 2.7.2 lists the ions (cation and. Lead Iv Oxide Cation.
From www.youtube.com
How to write chemical formula Lead OxideMolecular formula of Lead Lead Iv Oxide Cation There are obviously two types of ions, positive and negative, which are called cation and anion respectively. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Write the formula for lead (iv) oxide; All the anions are of this type, gaining the number of electrons. The charge of the ion is the number of protons. It. Lead Iv Oxide Cation.
From molekula.com
Purchase Lead (IV) oxide [1309600] online • Catalogue • Molekula Group Lead Iv Oxide Cation The charge of the ion is the number of protons. All the anions are of this type, gaining the number of electrons. Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total. Lead Iv Oxide Cation.
From slideplayer.com
OLD Naming Type I, Type II, Type III and Type IV Compounds ppt download Lead Iv Oxide Cation Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. It is also called lead dioxide, anhydrous plumbic. For each. Lead Iv Oxide Cation.
From www.researchgate.net
Ligandstabilized cations of germanium, tin and lead in oxidation state Lead Iv Oxide Cation The charge of the ion is the number of protons. Write the formula for lead (iv) oxide; Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid. Lead Iv Oxide Cation.
From www.shutterstock.com
Lead Iv Oxide Handwritten Chemical Formula Stock Illustration Lead Iv Oxide Cation It is also called lead dioxide, anhydrous plumbic. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. There are. Lead Iv Oxide Cation.
From www.slideserve.com
PPT Nomenclature of Compounds PowerPoint Presentation, free Lead Iv Oxide Cation Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. Write the symbol and charge of the cation (metal) first. Lead Iv Oxide Cation.
From www.pw.live
Lead IV Oxide Formula, Structure, Properties, Uses Lead Iv Oxide Cation There are obviously two types of ions, positive and negative, which are called cation and anion respectively. The charge of the ion is the number of protons. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. It is also called lead dioxide, anhydrous plumbic. All the anions are of this type, gaining the number. Lead Iv Oxide Cation.
From www.alamy.com
Lead (II,IV) oxide in Chemical Watch Glass on white laboratory table Lead Iv Oxide Cation Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Write the formula for lead (iv) oxide; Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. All the anions are of this type, gaining the number of electrons. It is also called lead dioxide, anhydrous plumbic.. Lead Iv Oxide Cation.
From apcpure.com
Lead (II,IV) Oxide (Red Lead) APC Pure Lead Iv Oxide Cation For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. It is also called lead dioxide, anhydrous plumbic. Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. There are. Lead Iv Oxide Cation.
From www.ck12.org
Ionic Compounds CK12 Foundation Lead Iv Oxide Cation Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Lead (iv) oxide also called plumbic oxide, anhydrous plumbic acid (sometimes wrongly called lead peroxide), though more. For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. It is also called lead dioxide, anhydrous plumbic. The charge. Lead Iv Oxide Cation.
From www.slideshare.net
Ionic bonding binary Lead Iv Oxide Cation There are obviously two types of ions, positive and negative, which are called cation and anion respectively. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second. Write the formula for lead (iv) oxide; For each p b4+ we need two negative. Lead Iv Oxide Cation.
From www.numerade.com
SOLVED[10 points] In water, solid manganese(Il) oxide reacts with Lead Iv Oxide Cation For each p b4+ we need two negative oxide ions o2− to balance out total positive and total negative charge. Pbo 2 is an oxide where the oxidation state of lead is +4 with the chemical name lead (iv) oxide. Figure 2.7.2 lists the ions (cation and anion) that have invariant oxidation states. All the anions are of this type,. Lead Iv Oxide Cation.