Lead Number Of Electrons Lost Or Gained . These two processes always occur together; Only larger atoms, such as lead and uranium, can typically carry larger charge. State the number of electrons lost or gained when the following elements form ions (state whether gained or lost): Oxidation is the loss of electrons by a molecule, atom, or ion. For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. Reduction is the gain of electrons. Determine number of electrons gained or lost. The original view of oxidation and reduction is that of adding or removing oxygen. F group 3a (3) cs i al An alternative view is to describe oxidation as the losing of. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. When one species is oxidized, another is. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant.
from www.slideserve.com
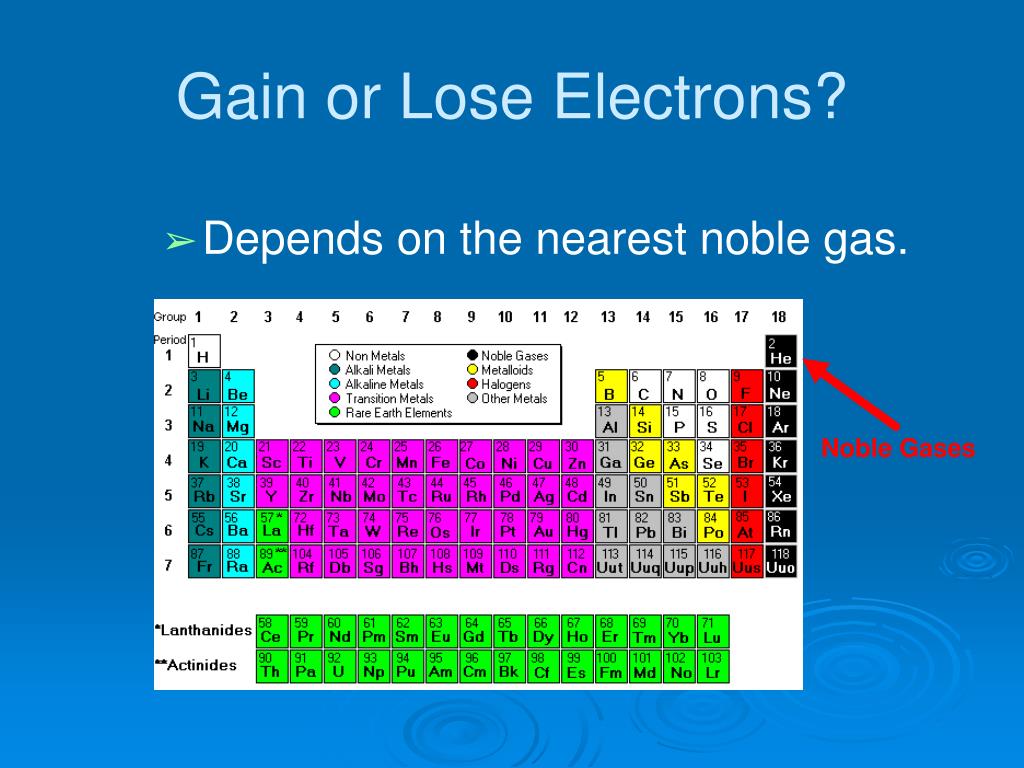
State the number of electrons lost or gained when the following elements form ions (state whether gained or lost): F group 3a (3) cs i al These two processes always occur together; An alternative view is to describe oxidation as the losing of. For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. The original view of oxidation and reduction is that of adding or removing oxygen. When one species is oxidized, another is. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. Reduction is the gain of electrons.
PPT CHEMICAL FORMULAS AND BONDING PowerPoint Presentation, free
Lead Number Of Electrons Lost Or Gained Oxidation is the loss of electrons by a molecule, atom, or ion. The original view of oxidation and reduction is that of adding or removing oxygen. State the number of electrons lost or gained when the following elements form ions (state whether gained or lost): For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. Reduction is the gain of electrons. An alternative view is to describe oxidation as the losing of. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Only larger atoms, such as lead and uranium, can typically carry larger charge. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. These two processes always occur together; Determine number of electrons gained or lost. Oxidation is the loss of electrons by a molecule, atom, or ion. When one species is oxidized, another is. F group 3a (3) cs i al
From www.numerade.com
SOLVED Determine the number of electrons lost or gained when each atom Lead Number Of Electrons Lost Or Gained F group 3a (3) cs i al An alternative view is to describe oxidation as the losing of. State the number of electrons lost or gained when the following elements form ions (state whether gained or lost): Only larger atoms, such as lead and uranium, can typically carry larger charge. Atoms tend to lose, gain, or share some valance electrons,. Lead Number Of Electrons Lost Or Gained.
From slideplayer.com
Oxidation Numbers. ppt download Lead Number Of Electrons Lost Or Gained For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained. Lead Number Of Electrons Lost Or Gained.
From www.nagwa.com
Question Video Finding the Number of Electrons Lost or Gained by an Lead Number Of Electrons Lost Or Gained These two processes always occur together; Reduction is the gain of electrons. F group 3a (3) cs i al When one species is oxidized, another is. For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. An alternative view is to describe oxidation as the losing of. Only larger atoms, such as. Lead Number Of Electrons Lost Or Gained.
From slideplayer.com
Elements and Ions. ppt download Lead Number Of Electrons Lost Or Gained An alternative view is to describe oxidation as the losing of. For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. F group 3a (3) cs i al These two processes always occur together; The original view of oxidation and reduction is that of adding or removing oxygen. Only larger atoms, such. Lead Number Of Electrons Lost Or Gained.
From www.slideserve.com
PPT CHEMICAL FORMULAS AND BONDING PowerPoint Presentation, free Lead Number Of Electrons Lost Or Gained Oxidation is the loss of electrons by a molecule, atom, or ion. These two processes always occur together; Only larger atoms, such as lead and uranium, can typically carry larger charge. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. The original view of oxidation and reduction. Lead Number Of Electrons Lost Or Gained.
From askfilo.com
The number of electrons lost or gained during the change, 3Fe+4H2 O→Fe3 O.. Lead Number Of Electrons Lost Or Gained Determine number of electrons gained or lost. Only larger atoms, such as lead and uranium, can typically carry larger charge. The original view of oxidation and reduction is that of adding or removing oxygen. These two processes always occur together; Reduction is the gain of electrons. In a balanced redox reaction, the number of electrons lost by the reductant equals. Lead Number Of Electrons Lost Or Gained.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5568233 Lead Number Of Electrons Lost Or Gained Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. F group 3a (3) cs i al State the number of electrons lost or gained when the following elements form ions (state whether gained or lost): Reduction is the gain of electrons. Oxidation is the loss of. Lead Number Of Electrons Lost Or Gained.
From www.slideserve.com
PPT Chapter 22 Chemical Bonds PowerPoint Presentation, free Lead Number Of Electrons Lost Or Gained Only larger atoms, such as lead and uranium, can typically carry larger charge. These two processes always occur together; In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. An. Lead Number Of Electrons Lost Or Gained.
From www.chegg.com
Solved State the number of electrons lost or gained when the Lead Number Of Electrons Lost Or Gained When one species is oxidized, another is. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Only larger atoms, such as lead and uranium, can typically carry larger charge. The original view of oxidation and reduction is that of adding or removing oxygen. Oxidation is the. Lead Number Of Electrons Lost Or Gained.
From www.numerade.com
SOLVED Indicate the number of electrons lost or gained when each of Lead Number Of Electrons Lost Or Gained For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. Oxidation is the loss of electrons by a molecule, atom, or ion. These two processes always occur together; In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. State the. Lead Number Of Electrons Lost Or Gained.
From utedzz.blogspot.com
Periodic Table Electrons Lost Or Gained Periodic Table Timeline Lead Number Of Electrons Lost Or Gained When one species is oxidized, another is. Oxidation is the loss of electrons by a molecule, atom, or ion. The original view of oxidation and reduction is that of adding or removing oxygen. Only larger atoms, such as lead and uranium, can typically carry larger charge. State the number of electrons lost or gained when the following elements form ions. Lead Number Of Electrons Lost Or Gained.
From www.slideserve.com
PPT UNIT 1 Structure and Function (Biochemistry) Chapter 2 Lead Number Of Electrons Lost Or Gained Oxidation is the loss of electrons by a molecule, atom, or ion. Only larger atoms, such as lead and uranium, can typically carry larger charge. The original view of oxidation and reduction is that of adding or removing oxygen. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the. Lead Number Of Electrons Lost Or Gained.
From www.nuclear-power.com
Lead Atomic Number Atomic Mass Density of Lead Lead Number Of Electrons Lost Or Gained These two processes always occur together; Determine number of electrons gained or lost. Oxidation is the loss of electrons by a molecule, atom, or ion. Reduction is the gain of electrons. Only larger atoms, such as lead and uranium, can typically carry larger charge. State the number of electrons lost or gained when the following elements form ions (state whether. Lead Number Of Electrons Lost Or Gained.
From www.slideserve.com
PPT Chapter 12 PowerPoint Presentation, free download ID2375980 Lead Number Of Electrons Lost Or Gained State the number of electrons lost or gained when the following elements form ions (state whether gained or lost): For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. When one species is oxidized, another is. In a balanced redox reaction, the number of electrons lost by the reductant equals the number. Lead Number Of Electrons Lost Or Gained.
From www.slideserve.com
PPT Recap Atomic Structure PowerPoint Presentation, free download Lead Number Of Electrons Lost Or Gained Reduction is the gain of electrons. When one species is oxidized, another is. Oxidation is the loss of electrons by a molecule, atom, or ion. F group 3a (3) cs i al Only larger atoms, such as lead and uranium, can typically carry larger charge. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the. Lead Number Of Electrons Lost Or Gained.
From www.vrogue.co
Calculate The Number Of Electrons Lost Or Gained Duri vrogue.co Lead Number Of Electrons Lost Or Gained Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. F group 3a (3) cs i al For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. Oxidation is the loss of electrons by a molecule, atom, or ion.. Lead Number Of Electrons Lost Or Gained.
From www.chegg.com
Solved 6.4 State the number of electrons lost or gained when Lead Number Of Electrons Lost Or Gained These two processes always occur together; Reduction is the gain of electrons. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. The original view of oxidation and reduction is that of adding or removing oxygen. For most elements under typical conditions, three electrons is the maximum number. Lead Number Of Electrons Lost Or Gained.
From www.chegg.com
Solved D. Determine the number of electrons lost or gained Lead Number Of Electrons Lost Or Gained The original view of oxidation and reduction is that of adding or removing oxygen. When one species is oxidized, another is. These two processes always occur together; State the number of electrons lost or gained when the following elements form ions (state whether gained or lost): F group 3a (3) cs i al Only larger atoms, such as lead and. Lead Number Of Electrons Lost Or Gained.
From www.slideserve.com
PPT Chemistry 120 PowerPoint Presentation, free download ID2089971 Lead Number Of Electrons Lost Or Gained Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. State the number of electrons lost or gained when the following elements form ions (state. Lead Number Of Electrons Lost Or Gained.
From cabinet.matttroy.net
Lead Periodic Table Electrons Matttroy Lead Number Of Electrons Lost Or Gained These two processes always occur together; State the number of electrons lost or gained when the following elements form ions (state whether gained or lost): An alternative view is to describe oxidation as the losing of. For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. Only larger atoms, such as lead. Lead Number Of Electrons Lost Or Gained.
From www.numerade.com
SOLVED Determine the number of electrons lost Or gained when each atom Lead Number Of Electrons Lost Or Gained Reduction is the gain of electrons. Determine number of electrons gained or lost. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. These two processes always occur together; For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. F. Lead Number Of Electrons Lost Or Gained.
From www.chegg.com
Solved State the number of electrons lost or gained when the Lead Number Of Electrons Lost Or Gained Only larger atoms, such as lead and uranium, can typically carry larger charge. Oxidation is the loss of electrons by a molecule, atom, or ion. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. For most elements under typical conditions, three electrons is the maximum number. Lead Number Of Electrons Lost Or Gained.
From www.numerade.com
SOLVED Determine the number of electrons lost O gained when each atom Lead Number Of Electrons Lost Or Gained In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. When one species is oxidized, another is. Determine number of electrons gained or lost. F. Lead Number Of Electrons Lost Or Gained.
From www.slideserve.com
PPT Chpt. 5 Chemical Bonding Chemical Formulas PowerPoint Lead Number Of Electrons Lost Or Gained State the number of electrons lost or gained when the following elements form ions (state whether gained or lost): In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. The original view of oxidation and reduction is that of adding or removing oxygen. Determine number of electrons gained. Lead Number Of Electrons Lost Or Gained.
From www.youtube.com
Determining the number of electrons lost or gained YouTube Lead Number Of Electrons Lost Or Gained An alternative view is to describe oxidation as the losing of. Oxidation is the loss of electrons by a molecule, atom, or ion. Only larger atoms, such as lead and uranium, can typically carry larger charge. Reduction is the gain of electrons. In a balanced redox reaction, the number of electrons lost by the reductant equals the number of electrons. Lead Number Of Electrons Lost Or Gained.
From www.vrogue.co
Calculate The Number Of Electrons Lost Or Gained Duri vrogue.co Lead Number Of Electrons Lost Or Gained When one species is oxidized, another is. An alternative view is to describe oxidation as the losing of. Only larger atoms, such as lead and uranium, can typically carry larger charge. Determine number of electrons gained or lost. These two processes always occur together; Oxidation is the loss of electrons by a molecule, atom, or ion. F group 3a (3). Lead Number Of Electrons Lost Or Gained.
From utedzz.blogspot.com
Periodic Table Electrons Lost Or Gained Periodic Table Timeline Lead Number Of Electrons Lost Or Gained Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. For most elements under typical conditions, three electrons is the maximum number that will be lost or gained. When one species is oxidized, another is. These two processes always occur together; Determine number of electrons gained or. Lead Number Of Electrons Lost Or Gained.
From www.numerade.com
SOLVED Use the periodic table and predict the number of electrons that Lead Number Of Electrons Lost Or Gained Reduction is the gain of electrons. An alternative view is to describe oxidation as the losing of. Only larger atoms, such as lead and uranium, can typically carry larger charge. When one species is oxidized, another is. Oxidation is the loss of electrons by a molecule, atom, or ion. State the number of electrons lost or gained when the following. Lead Number Of Electrons Lost Or Gained.
From www.slideserve.com
PPT Chapter 2 Atoms, Molecules, and Ions PowerPoint Presentation Lead Number Of Electrons Lost Or Gained When one species is oxidized, another is. An alternative view is to describe oxidation as the losing of. Only larger atoms, such as lead and uranium, can typically carry larger charge. State the number of electrons lost or gained when the following elements form ions (state whether gained or lost): For most elements under typical conditions, three electrons is the. Lead Number Of Electrons Lost Or Gained.
From www.youtube.com
4.7 Ions Losing & Gaining Electrons YouTube Lead Number Of Electrons Lost Or Gained These two processes always occur together; Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. An alternative view is to describe oxidation as the losing of. State the number of electrons lost or gained when the following elements form ions (state whether gained or lost): Determine. Lead Number Of Electrons Lost Or Gained.
From www.chegg.com
Solved dicate the number of electrons lost or gained when Lead Number Of Electrons Lost Or Gained F group 3a (3) cs i al State the number of electrons lost or gained when the following elements form ions (state whether gained or lost): Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. The original view of oxidation and reduction is that of adding. Lead Number Of Electrons Lost Or Gained.
From www.slideshare.net
Understanding The Chemistry Of Atoms to Ions Lead Number Of Electrons Lost Or Gained Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Determine number of electrons gained or lost. These two processes always occur together; Oxidation is the loss of electrons by a molecule, atom, or ion. Only larger atoms, such as lead and uranium, can typically carry larger. Lead Number Of Electrons Lost Or Gained.
From slideplayer.com
4/3/2019 Periodic Table L. Relyea. ppt download Lead Number Of Electrons Lost Or Gained An alternative view is to describe oxidation as the losing of. Only larger atoms, such as lead and uranium, can typically carry larger charge. Reduction is the gain of electrons. When one species is oxidized, another is. Oxidation is the loss of electrons by a molecule, atom, or ion. Atoms tend to lose, gain, or share some valance electrons, making. Lead Number Of Electrons Lost Or Gained.
From www.chegg.com
Solved Determine the number of electrons lost or gained when Lead Number Of Electrons Lost Or Gained When one species is oxidized, another is. State the number of electrons lost or gained when the following elements form ions (state whether gained or lost): Determine number of electrons gained or lost. These two processes always occur together; F group 3a (3) cs i al Reduction is the gain of electrons. The original view of oxidation and reduction is. Lead Number Of Electrons Lost Or Gained.
From www.chegg.com
Solved [References) Indicate the number of electrons lost or Lead Number Of Electrons Lost Or Gained These two processes always occur together; Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron configuration of the nearest noble gas, i.e.,. Reduction is the gain of electrons. Determine number of electrons gained or lost. The original view of oxidation and reduction is that of adding or removing oxygen. For most elements under. Lead Number Of Electrons Lost Or Gained.