Do Atom Have Positive Charge . If an atom loses one or more electrons, it becomes a positively charged ion; The electrons are negatively charged, and this opposing charge is what binds. You can use this table to predict whether an atom can bond with another atom. This is because they contain equal numbers of positive protons and negative electrons. Every atom has no overall charge (neutral). Protons have a positive electrical charge of one (+1) (+ 1) and a mass of 1 atomic mass unit (amu) (amu), which is about 1.67. If an atom gains one or more electrons, it becomes a. Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. 93 rows this table shows the most common charges for atoms of the chemical elements.
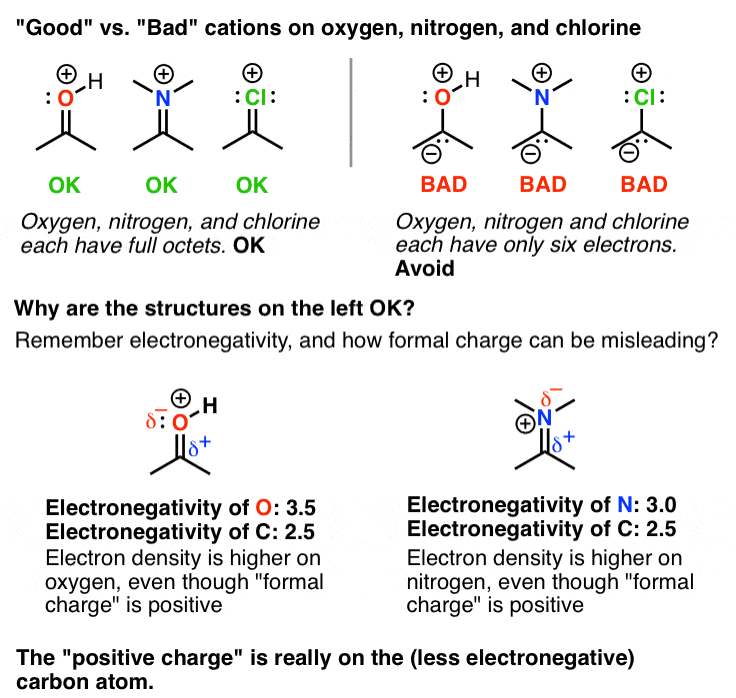
from www.masterorganicchemistry.com
This is because they contain equal numbers of positive protons and negative electrons. The electrons are negatively charged, and this opposing charge is what binds. If an atom loses one or more electrons, it becomes a positively charged ion; You can use this table to predict whether an atom can bond with another atom. Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. If an atom gains one or more electrons, it becomes a. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electrical charge of one (+1) (+ 1) and a mass of 1 atomic mass unit (amu) (amu), which is about 1.67. Every atom has no overall charge (neutral). 93 rows this table shows the most common charges for atoms of the chemical elements.
Evaluating Resonance Structures With Positive Charge
Do Atom Have Positive Charge If an atom gains one or more electrons, it becomes a. You can use this table to predict whether an atom can bond with another atom. This is because they contain equal numbers of positive protons and negative electrons. Protons have a positive electrical charge of one (+1) (+ 1) and a mass of 1 atomic mass unit (amu) (amu), which is about 1.67. Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. The electrons are negatively charged, and this opposing charge is what binds. If an atom gains one or more electrons, it becomes a. If an atom loses one or more electrons, it becomes a positively charged ion; 93 rows this table shows the most common charges for atoms of the chemical elements. Every atom has no overall charge (neutral). Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation ID2508414 Do Atom Have Positive Charge Every atom has no overall charge (neutral). This is because they contain equal numbers of positive protons and negative electrons. You can use this table to predict whether an atom can bond with another atom. 93 rows this table shows the most common charges for atoms of the chemical elements. If an atom gains one or more electrons, it becomes. Do Atom Have Positive Charge.
From calebcroomphysci4dummies.weebly.com
Atomic Structure Physical Science For Dummies Do Atom Have Positive Charge If an atom gains one or more electrons, it becomes a. Every atom has no overall charge (neutral). You can use this table to predict whether an atom can bond with another atom. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electrical. Do Atom Have Positive Charge.
From www.expii.com
Atoms — Definition & Overview Expii Do Atom Have Positive Charge Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. Every atom has no overall charge (neutral). Protons have a positive electrical charge of one (+1) (+ 1) and a mass of 1 atomic mass unit (amu) (amu), which is about 1.67. You can use this table to predict whether an atom can. Do Atom Have Positive Charge.
From www.mindomo.com
Energy Mind Map Do Atom Have Positive Charge 93 rows this table shows the most common charges for atoms of the chemical elements. If an atom loses one or more electrons, it becomes a positively charged ion; This is because they contain equal numbers of positive protons and negative electrons. The electrons are negatively charged, and this opposing charge is what binds. Protons have a positive electrical charge. Do Atom Have Positive Charge.
From electricallive.com
Atomic Structure And Electric Charge Electrical engineering interview Do Atom Have Positive Charge Protons have a positive electrical charge of one (+1) (+ 1) and a mass of 1 atomic mass unit (amu) (amu), which is about 1.67. Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. You can use this table to predict whether an atom can bond with another atom. 93 rows this. Do Atom Have Positive Charge.
From www.chemistrystudent.com
Atomic Structure (ALevel) ChemistryStudent Do Atom Have Positive Charge The electrons are negatively charged, and this opposing charge is what binds. If an atom gains one or more electrons, it becomes a. This is because they contain equal numbers of positive protons and negative electrons. Every atom has no overall charge (neutral). You can use this table to predict whether an atom can bond with another atom. Protons have. Do Atom Have Positive Charge.
From www.masterorganicchemistry.com
Evaluating Resonance Structures With Positive Charge Do Atom Have Positive Charge The electrons are negatively charged, and this opposing charge is what binds. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. Every atom has no overall charge (neutral). You can. Do Atom Have Positive Charge.
From www.slideserve.com
PPT What part of an atom is the arrow pointing to? PowerPoint Do Atom Have Positive Charge You can use this table to predict whether an atom can bond with another atom. Every atom has no overall charge (neutral). This is because they contain equal numbers of positive protons and negative electrons. Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. Protons have a positive electrical charge of one. Do Atom Have Positive Charge.
From quizmouvemente.z21.web.core.windows.net
Are Electrons Attracted To Positive Charges Do Atom Have Positive Charge If an atom gains one or more electrons, it becomes a. If an atom loses one or more electrons, it becomes a positively charged ion; Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. 93 rows this table shows the most common charges for atoms of the. Do Atom Have Positive Charge.
From lessonschoolquintuples.z5.web.core.windows.net
How To Ions Form Do Atom Have Positive Charge If an atom gains one or more electrons, it becomes a. If an atom loses one or more electrons, it becomes a positively charged ion; This is because they contain equal numbers of positive protons and negative electrons. You can use this table to predict whether an atom can bond with another atom. Protons have a positive electrical charge of. Do Atom Have Positive Charge.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation Do Atom Have Positive Charge If an atom loses one or more electrons, it becomes a positively charged ion; 93 rows this table shows the most common charges for atoms of the chemical elements. Protons have a positive electrical charge of one (+1) (+ 1) and a mass of 1 atomic mass unit (amu) (amu), which is about 1.67. Protons have a positive electric charge. Do Atom Have Positive Charge.
From slideplayer.com
Lesson objectives; You will learn ppt download Do Atom Have Positive Charge Every atom has no overall charge (neutral). If an atom loses one or more electrons, it becomes a positively charged ion; This is because they contain equal numbers of positive protons and negative electrons. If an atom gains one or more electrons, it becomes a. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of. Do Atom Have Positive Charge.
From peyton-has-tapia.blogspot.com
An Atom With a Net Positive Charge Must Have More PeytonhasTapia Do Atom Have Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. This is because they contain equal numbers of positive protons and negative electrons. The electrons are negatively charged, and this opposing. Do Atom Have Positive Charge.
From telgurus.co.uk
What is the overall charge of the nucleus of an atom and why? Do Atom Have Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. You can use this table to predict whether an atom can bond with another atom. Protons have a positive electrical charge of one (+1) (+ 1) and a mass of 1 atomic mass unit (amu) (amu), which is. Do Atom Have Positive Charge.
From www.examanalysis.in
Electric Current Do Atom Have Positive Charge This is because they contain equal numbers of positive protons and negative electrons. Every atom has no overall charge (neutral). Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. You. Do Atom Have Positive Charge.
From www.slideserve.com
PPT Lecture 1 Charge and Coulomb’s Law PowerPoint Presentation, free Do Atom Have Positive Charge 93 rows this table shows the most common charges for atoms of the chemical elements. Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. Protons have a positive electrical charge of one (+1) (+ 1) and a mass of 1 atomic mass unit (amu) (amu), which is about 1.67. This is because. Do Atom Have Positive Charge.
From www.nagwa.com
Question Video Recalling the Name of the Positively Charged Particles Do Atom Have Positive Charge Every atom has no overall charge (neutral). Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. 93 rows this table shows the most common charges for atoms of the chemical elements. You can use this table to predict whether an atom can bond with another atom. If. Do Atom Have Positive Charge.
From circuitpennhockey01yh.z13.web.core.windows.net
Atom With Electrical Charge Do Atom Have Positive Charge 93 rows this table shows the most common charges for atoms of the chemical elements. Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. You can use this table to. Do Atom Have Positive Charge.
From slideplayer.com
Atomic structure Mrs. Meadows. ppt download Do Atom Have Positive Charge You can use this table to predict whether an atom can bond with another atom. If an atom gains one or more electrons, it becomes a. If an atom loses one or more electrons, it becomes a positively charged ion; Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left(. Do Atom Have Positive Charge.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Do Atom Have Positive Charge The electrons are negatively charged, and this opposing charge is what binds. This is because they contain equal numbers of positive protons and negative electrons. If an atom gains one or more electrons, it becomes a. You can use this table to predict whether an atom can bond with another atom. Protons have a positive electrical charge of one \(\left(. Do Atom Have Positive Charge.
From www.atlearner.com
What is Electric Charge? (1 Electrostatics) Atlearner Learn Science Do Atom Have Positive Charge Protons have a positive electrical charge of one (+1) (+ 1) and a mass of 1 atomic mass unit (amu) (amu), which is about 1.67. If an atom loses one or more electrons, it becomes a positively charged ion; The electrons are negatively charged, and this opposing charge is what binds. If an atom gains one or more electrons, it. Do Atom Have Positive Charge.
From slideplayer.com
Ions and Ionic Compounds ppt download Do Atom Have Positive Charge You can use this table to predict whether an atom can bond with another atom. Every atom has no overall charge (neutral). This is because they contain equal numbers of positive protons and negative electrons. 93 rows this table shows the most common charges for atoms of the chemical elements. If an atom loses one or more electrons, it becomes. Do Atom Have Positive Charge.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation ID2508414 Do Atom Have Positive Charge The electrons are negatively charged, and this opposing charge is what binds. Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. Every atom has no overall charge (neutral). If an atom gains one or more electrons, it becomes a. You can use this table to predict whether an atom can bond with. Do Atom Have Positive Charge.
From www.slideserve.com
PPT Chapter 21 Electric Charge and Electric Fields PowerPoint Do Atom Have Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. The electrons are negatively charged, and this opposing charge is what binds. 93 rows this table shows the most common charges for atoms of the chemical elements. Every atom has no overall charge (neutral). Protons have a positive. Do Atom Have Positive Charge.
From www.sliderbase.com
Formula of Ionic Compounds Do Atom Have Positive Charge Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. Protons have a positive electrical charge of one (+1) (+ 1) and a mass of 1 atomic mass unit (amu) (amu), which is about 1.67. If an atom gains one or more electrons, it becomes a. The electrons are negatively charged, and this. Do Atom Have Positive Charge.
From jpmdmlriiu.blogspot.com
How To Tell The Charge Of An Atom The amount of electrons and protons Do Atom Have Positive Charge 93 rows this table shows the most common charges for atoms of the chemical elements. The electrons are negatively charged, and this opposing charge is what binds. Protons have a positive electrical charge of one (+1) (+ 1) and a mass of 1 atomic mass unit (amu) (amu), which is about 1.67. Protons have a positive electrical charge of one. Do Atom Have Positive Charge.
From periodictable.me
What are the Difference Between Charge and Electron? Do Atom Have Positive Charge Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. 93 rows this table shows the most common charges for atoms of the chemical elements. If an atom loses one or more electrons, it becomes a positively charged ion; Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass. Do Atom Have Positive Charge.
From www.sliderbase.com
Atoms and the Periodic table Presentation Chemistry Do Atom Have Positive Charge If an atom gains one or more electrons, it becomes a. The electrons are negatively charged, and this opposing charge is what binds. This is because they contain equal numbers of positive protons and negative electrons. Every atom has no overall charge (neutral). Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged.. Do Atom Have Positive Charge.
From learn.sparkfun.com
What is Electricity? SparkFun Learn Do Atom Have Positive Charge You can use this table to predict whether an atom can bond with another atom. The electrons are negatively charged, and this opposing charge is what binds. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. This is because they contain equal numbers of positive protons and. Do Atom Have Positive Charge.
From www.shalom-education.com
Atomic Structure and Ions GCSE Chemistry Revision Do Atom Have Positive Charge If an atom gains one or more electrons, it becomes a. Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. If an atom loses one or more electrons, it becomes a positively charged ion; The electrons are negatively charged, and this opposing charge is what binds. 93 rows this table shows the. Do Atom Have Positive Charge.
From www.masterorganicchemistry.com
7 Factors That Stabilize Positive Charge in Organic Chemistry Master Do Atom Have Positive Charge You can use this table to predict whether an atom can bond with another atom. The electrons are negatively charged, and this opposing charge is what binds. If an atom gains one or more electrons, it becomes a. Every atom has no overall charge (neutral). Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of. Do Atom Have Positive Charge.
From www.masterorganicchemistry.com
7 Factors That Stabilize Positive Charge in Organic Chemistry Master Do Atom Have Positive Charge This is because they contain equal numbers of positive protons and negative electrons. Protons have a positive electrical charge of one (+1) (+ 1) and a mass of 1 atomic mass unit (amu) (amu), which is about 1.67. Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),.. Do Atom Have Positive Charge.
From www.sciencefacts.net
Atom Definition, Structure & Parts with Labeled Diagram Do Atom Have Positive Charge Protons have a positive electrical charge of one \(\left( +1 \right)\) and a mass of 1 atomic mass unit \(\left( \text{amu} \right)\),. If an atom loses one or more electrons, it becomes a positively charged ion; If an atom gains one or more electrons, it becomes a. Protons have a positive electric charge and neutrons have no charge, so the. Do Atom Have Positive Charge.
From www.slideserve.com
PPT Protons are positive (+) charged particles found in the nucleus Do Atom Have Positive Charge You can use this table to predict whether an atom can bond with another atom. If an atom loses one or more electrons, it becomes a positively charged ion; If an atom gains one or more electrons, it becomes a. Protons have a positive electric charge and neutrons have no charge, so the nucleus is positively charged. 93 rows this. Do Atom Have Positive Charge.
From sciencenotes.org
Learn the Parts of an Atom Do Atom Have Positive Charge You can use this table to predict whether an atom can bond with another atom. 93 rows this table shows the most common charges for atoms of the chemical elements. Every atom has no overall charge (neutral). Protons have a positive electrical charge of one (+1) (+ 1) and a mass of 1 atomic mass unit (amu) (amu), which is. Do Atom Have Positive Charge.