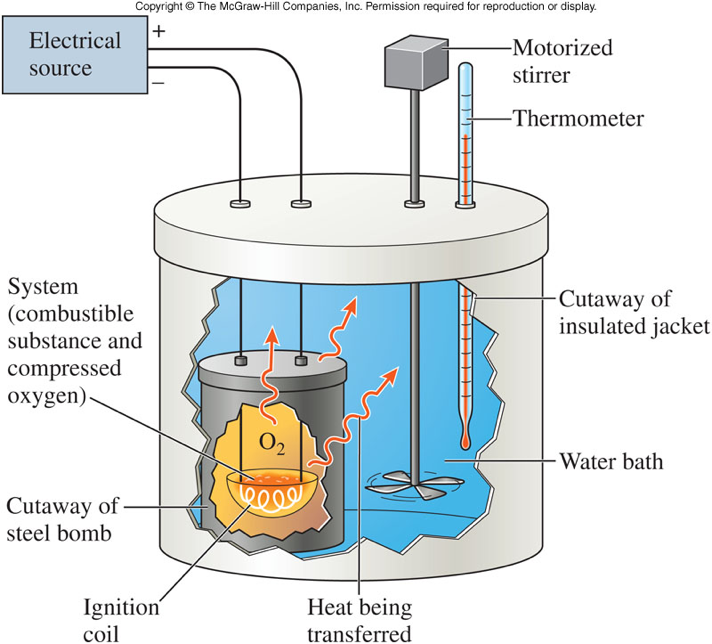
Calorimeter How Does It Work . To do so, the heat is exchanged with a calibrated object. a calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. apply the first law of thermodynamics to calorimetry. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Compare heat flow from hot to cold objects in an ideal calorimeter.
from shaunmwilliams.com
apply the first law of thermodynamics to calorimetry. calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. To do so, the heat is exchanged with a calibrated object. a calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal calorimeter. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for.
Chapter 6 Presentation
Calorimeter How Does It Work apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter. apply the first law of thermodynamics to calorimetry. a calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter How Does It Work calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Compare heat flow from hot to cold objects in an ideal calorimeter. a calorimeter measures the heat transferred to or from an object during a chemical or. Calorimeter How Does It Work.
From www.tffn.net
How Does a Calorimeter Work? An Indepth Guide The Enlightened Mindset Calorimeter How Does It Work calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged. Calorimeter How Does It Work.
From exyxwauhc.blob.core.windows.net
What Is Calorimetry And How Does It Work at Jesse Mignone blog Calorimeter How Does It Work calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. apply the first law of thermodynamics to calorimetry. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Compare heat flow from hot to cold objects in an ideal calorimeter. calorimetry is. Calorimeter How Does It Work.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimeter How Does It Work apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter. To do so, the heat is exchanged with a calibrated object. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. calorimetry measures enthalpy changes during chemical processes, where the magnitude. Calorimeter How Does It Work.
From exyqpgrbs.blob.core.windows.net
Calorimetry Equation Explained at Sandra Roof blog Calorimeter How Does It Work calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry measures enthalpy changes during chemical. Calorimeter How Does It Work.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimeter How Does It Work calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. Compare heat flow from hot to cold objects in an ideal calorimeter. To do so, the heat is exchanged with a calibrated object. . Calorimeter How Does It Work.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter How Does It Work calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal calorimeter. calorimetry measures enthalpy changes during chemical processes, where the magnitude of. Calorimeter How Does It Work.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle and Structure of Calorimeter How Does It Work calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you. calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. apply the first. Calorimeter How Does It Work.
From users.highland.edu
Calorimetry Calorimeter How Does It Work calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. a calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you. calorimetry is used to measure amounts of heat transferred to or from a substance. apply the first. Calorimeter How Does It Work.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter How Does It Work Compare heat flow from hot to cold objects in an ideal calorimeter. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is used to measure quantities of heat, and can be used to determine the heat of. Calorimeter How Does It Work.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tecscience Calorimeter How Does It Work calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. To do so, the heat is exchanged with a calibrated object. Compare heat flow from hot to cold objects in an ideal calorimeter. calorimetry is used to measure amounts of heat transferred to or from a substance. . Calorimeter How Does It Work.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube Calorimeter How Does It Work apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter. To do so, the heat is exchanged with a calibrated object. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. calorimetry is used to measure amounts of heat transferred to. Calorimeter How Does It Work.
From www.shaalaa.com
Explain the construction of a calorimeter. Draw the necessary figure. Science Calorimeter How Does It Work Compare heat flow from hot to cold objects in an ideal calorimeter. a calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you. apply the first law of thermodynamics to calorimetry. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is used. Calorimeter How Does It Work.
From thermonine92.blogspot.com
Thermochemistry Calorimeter Calorimeter How Does It Work calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you. apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter. calorimetry measures enthalpy. Calorimeter How Does It Work.
From www.researchgate.net
Schematic diagram of the calorimeter used in this work Download Scientific Diagram Calorimeter How Does It Work calorimetry is used to measure amounts of heat transferred to or from a substance. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Compare heat flow from hot to cold objects in an ideal calorimeter. a calorimeter measures the heat transferred to or from an object during a chemical or. Calorimeter How Does It Work.
From www.youtube.com
Constructing a Calorimeter YouTube Calorimeter How Does It Work Compare heat flow from hot to cold objects in an ideal calorimeter. a calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you. calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. apply the first law of thermodynamics. Calorimeter How Does It Work.
From shaunmwilliams.com
Chapter 6 Presentation Calorimeter How Does It Work calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. a calorimeter measures the heat transferred to or from an. Calorimeter How Does It Work.
From www.slideserve.com
PPT a “ Calorimeter ” PowerPoint Presentation, free download ID7050684 Calorimeter How Does It Work calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimeter, device for measuring the heat developed during. Calorimeter How Does It Work.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeters Calorimeter How Does It Work calorimetry is used to measure amounts of heat transferred to or from a substance. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. a calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you. To do so, the heat is exchanged with. Calorimeter How Does It Work.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter How Does It Work calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from hot to cold objects in an ideal calorimeter. calorimetry is used to measure quantities of heat, and can be used to determine the heat of. Calorimeter How Does It Work.
From www.embibe.com
Explain the construction of a calorimeter Draw the necessary figure Calorimeter How Does It Work calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. Compare heat flow from hot to cold objects in an ideal calorimeter. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is used to measure amounts of heat transferred to or. Calorimeter How Does It Work.
From ddscalorimeters.com
How does a bomb calorimeter work? Calorimeter How Does It Work calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. apply the first law of thermodynamics to calorimetry. a calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature. Calorimeter How Does It Work.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimeters Calorimeter How Does It Work Compare heat flow from hot to cold objects in an ideal calorimeter. apply the first law of thermodynamics to calorimetry. To do so, the heat is exchanged with a calibrated object. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. a calorimeter measures the heat transferred to or from an. Calorimeter How Does It Work.
From hxentbteo.blob.core.windows.net
Calorimeter Experiment Calculations at Jerry Obrien blog Calorimeter How Does It Work calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. To do so, the heat is exchanged with a calibrated object. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Compare heat flow from hot to cold objects in an ideal calorimeter. apply the first law. Calorimeter How Does It Work.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimeter How Does It Work Compare heat flow from hot to cold objects in an ideal calorimeter. calorimetry is used to measure amounts of heat transferred to or from a substance. apply the first law of thermodynamics to calorimetry. a calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you. calorimetry is used. Calorimeter How Does It Work.
From www.tffn.net
How Does a Calorimeter Work? An Indepth Guide The Enlightened Mindset Calorimeter How Does It Work calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. To do so, the heat is exchanged with a calibrated object. apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter. calorimetry is used to measure amounts. Calorimeter How Does It Work.
From ddscalorimeters.com
How does a bomb calorimeter work? Calorimeter How Does It Work calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. apply the first law of thermodynamics to calorimetry. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a substance. Compare heat flow from. Calorimeter How Does It Work.
From saylordotorg.github.io
Calorimetry Calorimeter How Does It Work calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. Compare heat flow from hot to cold objects in an ideal calorimeter. apply the first law of thermodynamics to calorimetry. To do so,. Calorimeter How Does It Work.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimeter How Does It Work To do so, the heat is exchanged with a calibrated object. a calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you. Compare heat flow from hot to cold objects in an ideal calorimeter. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for.. Calorimeter How Does It Work.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimeter How Does It Work calorimetry is used to measure amounts of heat transferred to or from a substance. apply the first law of thermodynamics to calorimetry. To do so, the heat is exchanged with a calibrated object. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimeter, device for measuring the heat developed during a. Calorimeter How Does It Work.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation & Application Calorimeter How Does It Work calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Compare heat flow from hot to cold objects in an ideal calorimeter. calorimetry is used to measure quantities of heat, and can be used to determine the. Calorimeter How Does It Work.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types, Examples and Uses CollegeSearch Calorimeter How Does It Work calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. To do so, the heat is exchanged with a calibrated object. Compare heat flow from hot to cold objects in an ideal calorimeter. a calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you. calorimetry. Calorimeter How Does It Work.
From www.tffn.net
How Does a Calorimeter Work? An Indepth Guide The Enlightened Mindset Calorimeter How Does It Work calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you. calorimeter, device for. Calorimeter How Does It Work.
From exyqpgrbs.blob.core.windows.net
Calorimetry Equation Explained at Sandra Roof blog Calorimeter How Does It Work a calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you. calorimetry is used to measure amounts of heat transferred to or from a substance. apply the first law of thermodynamics to calorimetry. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. . Calorimeter How Does It Work.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimeter How Does It Work calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter measures the heat transferred to or from an object during a chemical or physical process, and you. calorimetry is used to measure quantities of heat, and can be used to determine the heat of a reaction through. calorimeter, device for. Calorimeter How Does It Work.