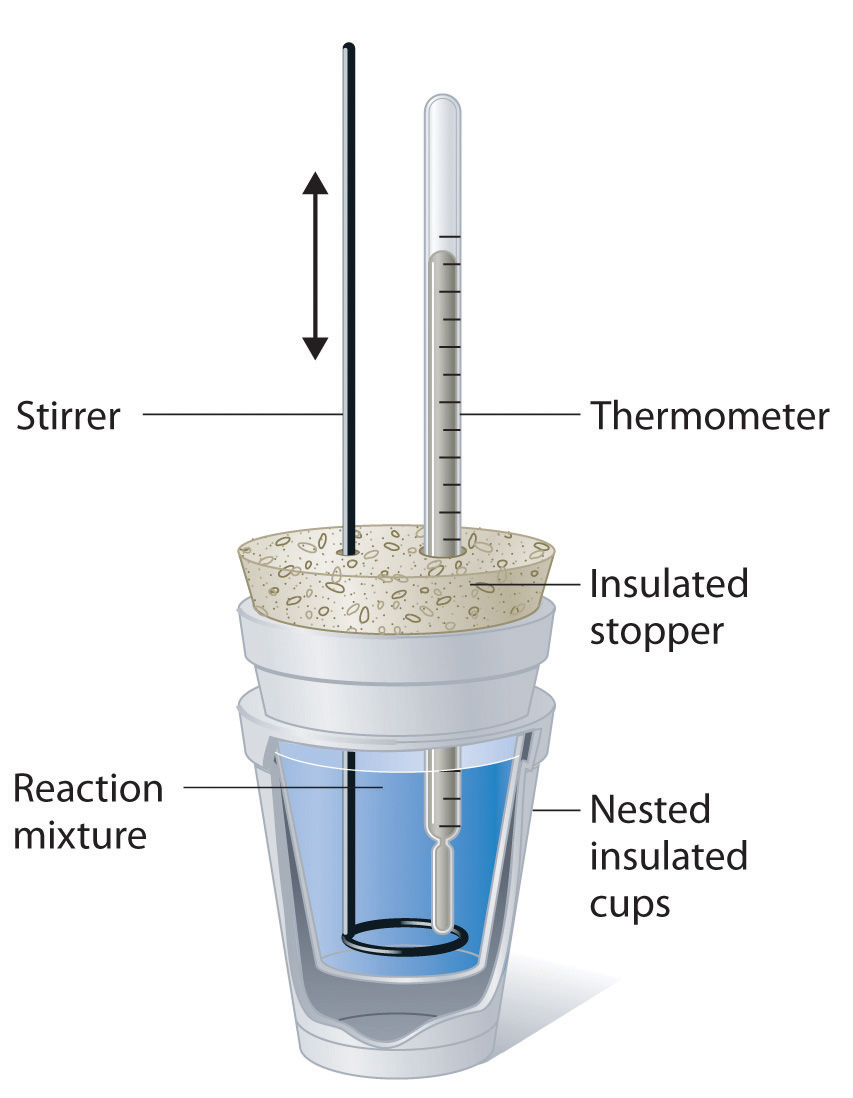
Hypothesis Calorimetry Lab . lab session 9, experiment 8: Use hess’s law to determine. Find the heat capacity (cp) of a calorimeter and contents (calibration). Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Determine the hrxn, the enthalpy of. show how these equations must be summed together according to hess’s law to determine δh δ h for the combustion of iron (target equation. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. the heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. to measure thermochemical properties of aqueous reactions and substances using a simple calorimeter. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. this experiment has three primary objectives:
from chem.libretexts.org
calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. to measure thermochemical properties of aqueous reactions and substances using a simple calorimeter. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. this experiment has three primary objectives: Find the heat capacity (cp) of a calorimeter and contents (calibration). in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. lab session 9, experiment 8: Use hess’s law to determine. Determine the hrxn, the enthalpy of. the heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the.
6.3 Calorimetry Chemistry LibreTexts
Hypothesis Calorimetry Lab Find the heat capacity (cp) of a calorimeter and contents (calibration). in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Find the heat capacity (cp) of a calorimeter and contents (calibration). Determine the hrxn, the enthalpy of. lab session 9, experiment 8: this experiment has three primary objectives: the heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. show how these equations must be summed together according to hess’s law to determine δh δ h for the combustion of iron (target equation. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. to measure thermochemical properties of aqueous reactions and substances using a simple calorimeter. Use hess’s law to determine.
From hxevytssw.blob.core.windows.net
Calorimeter Experiment Analysis at Brenda Maxwell blog Hypothesis Calorimetry Lab in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. Determine the hrxn, the enthalpy of. Find the heat capacity (cp) of a calorimeter and contents (calibration). to measure thermochemical properties of aqueous reactions and substances using a simple calorimeter. this experiment has three primary. Hypothesis Calorimetry Lab.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Hypothesis Calorimetry Lab in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. Find the heat capacity (cp) of a calorimeter and contents (calibration). lab session 9, experiment 8: Use hess’s law to determine. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample. Hypothesis Calorimetry Lab.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry experiment. An example abstract Hypothesis Calorimetry Lab Determine the hrxn, the enthalpy of. to measure thermochemical properties of aqueous reactions and substances using a simple calorimeter. Find the heat capacity (cp) of a calorimeter and contents (calibration). Use hess’s law to determine. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. lab session 9, experiment 8: . Hypothesis Calorimetry Lab.
From www.slideshare.net
Calorimetry Lab Hypothesis Calorimetry Lab to measure thermochemical properties of aqueous reactions and substances using a simple calorimeter. this experiment has three primary objectives: lab session 9, experiment 8: the heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. show how these equations must be summed together according. Hypothesis Calorimetry Lab.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Hypothesis Calorimetry Lab the heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. in this experiment you will heat a known mass of a metal to. Hypothesis Calorimetry Lab.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Hypothesis Calorimetry Lab show how these equations must be summed together according to hess’s law to determine δh δ h for the combustion of iron (target equation. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. this experiment has three primary objectives: in this experiment you will heat a known mass of. Hypothesis Calorimetry Lab.
From studylib.net
Lab Calorimetry High School Science Help Hypothesis Calorimetry Lab show how these equations must be summed together according to hess’s law to determine δh δ h for the combustion of iron (target equation. this experiment has three primary objectives: lab session 9, experiment 8: to measure thermochemical properties of aqueous reactions and substances using a simple calorimeter. in this experiment you will heat a. Hypothesis Calorimetry Lab.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Hypothesis Calorimetry Lab in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. this experiment has three primary objectives: show how these equations must be. Hypothesis Calorimetry Lab.
From hxevytssw.blob.core.windows.net
Calorimeter Experiment Analysis at Brenda Maxwell blog Hypothesis Calorimetry Lab to measure thermochemical properties of aqueous reactions and substances using a simple calorimeter. Determine the hrxn, the enthalpy of. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Use hess’s law to determine. Specific heat is an intensive property of a single phase (solid, liquid. Hypothesis Calorimetry Lab.
From www.studypool.com
SOLUTION Student exploration calorimetry lab Studypool Hypothesis Calorimetry Lab this experiment has three primary objectives: Use hess’s law to determine. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. to measure thermochemical properties of aqueous reactions and substances using a simple calorimeter. the heat of neutralization that is lost in the chemical reaction (the system) is gained by. Hypothesis Calorimetry Lab.
From www.scribd.com
Calorimetry Experiment Lab Report PDF Sodium Hydroxide Hydrochloric Acid Hypothesis Calorimetry Lab Use hess’s law to determine. lab session 9, experiment 8: calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. to measure thermochemical properties of aqueous reactions and substances using a simple calorimeter. show how these equations must be summed together according to hess’s. Hypothesis Calorimetry Lab.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Hypothesis Calorimetry Lab show how these equations must be summed together according to hess’s law to determine δh δ h for the combustion of iron (target equation. lab session 9, experiment 8: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. this experiment has three primary objectives: to measure thermochemical properties. Hypothesis Calorimetry Lab.
From studylib.net
calorimetry lab Hypothesis Calorimetry Lab the heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. show how these equations must be summed together according to hess’s law to. Hypothesis Calorimetry Lab.
From giobxedsi.blob.core.windows.net
Calorimeter Experiment Calculation at Mabel Ballenger blog Hypothesis Calorimetry Lab Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. Determine the hrxn, the enthalpy of. show how these equations must be summed together according to hess’s law to. Hypothesis Calorimetry Lab.
From www.nagwa.com
Question Video Determining the Suitability of Experimental Apparatus for a Calorimetry Hypothesis Calorimetry Lab calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. to measure thermochemical properties of aqueous reactions and substances using a simple calorimeter. Find the heat capacity (cp) of a calorimeter and contents (calibration). lab session 9, experiment 8: Specific heat is an intensive property. Hypothesis Calorimetry Lab.
From chem.libretexts.org
6.3 Calorimetry Chemistry LibreTexts Hypothesis Calorimetry Lab lab session 9, experiment 8: calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. to measure thermochemical properties of aqueous reactions and substances using a simple calorimeter. Determine the hrxn, the enthalpy of. this experiment has three primary objectives: Use hess’s law to. Hypothesis Calorimetry Lab.
From hxekemegs.blob.core.windows.net
Calorimetry Chemistry Experiment at Robert Montague blog Hypothesis Calorimetry Lab show how these equations must be summed together according to hess’s law to determine δh δ h for the combustion of iron (target equation. the heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. Determine the hrxn, the enthalpy of. in this experiment you will. Hypothesis Calorimetry Lab.
From www.studypool.com
SOLUTION Student exploration calorimetry lab vocabulary calorie calorimeter joule specific heat Hypothesis Calorimetry Lab show how these equations must be summed together according to hess’s law to determine δh δ h for the combustion of iron (target equation. to measure thermochemical properties of aqueous reactions and substances using a simple calorimeter. Find the heat capacity (cp) of a calorimeter and contents (calibration). Determine the hrxn, the enthalpy of. the heat of. Hypothesis Calorimetry Lab.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity Hypothesis Calorimetry Lab Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. show how these equations must be summed together according to hess’s law to determine δh δ h for the. Hypothesis Calorimetry Lab.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Docsity Hypothesis Calorimetry Lab this experiment has three primary objectives: the heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. to measure thermochemical properties of aqueous reactions and substances using a simple calorimeter. Find the heat capacity (cp) of a calorimeter and contents (calibration). show how these equations. Hypothesis Calorimetry Lab.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Hypothesis Calorimetry Lab Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Find the heat capacity (cp) of a calorimeter and contents (calibration). Determine the hrxn, the enthalpy of. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. lab session. Hypothesis Calorimetry Lab.
From studylib.net
experiment 9 Thermochemistry Calorimetry Hypothesis Calorimetry Lab calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Use hess’s law to determine. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. Specific heat is an intensive property of a single. Hypothesis Calorimetry Lab.
From studylib.net
Calorimetry Experiment and Practice Problems for Virtual Lab Key Hypothesis Calorimetry Lab to measure thermochemical properties of aqueous reactions and substances using a simple calorimeter. this experiment has three primary objectives: show how these equations must be summed together according to hess’s law to determine δh δ h for the combustion of iron (target equation. Use hess’s law to determine. calorimetry is the study of heat transferred in. Hypothesis Calorimetry Lab.
From pdfprof.com
experiment 25 calorimetry lab report Hypothesis Calorimetry Lab Determine the hrxn, the enthalpy of. the heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. Use hess’s law to determine. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. Find the heat. Hypothesis Calorimetry Lab.
From studylib.net
Calorimetry Lab Specific Heat Capacity Hypothesis Calorimetry Lab calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. the heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. in this experiment you will heat a known mass of a metal to. Hypothesis Calorimetry Lab.
From www.studypool.com
SOLUTION Calorimetry Lab Studypool Hypothesis Calorimetry Lab Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. show how these equations must be summed together according to hess’s law to determine δh δ h for the combustion of iron (target equation. in this experiment you will heat a known mass of a metal to a known temperature and. Hypothesis Calorimetry Lab.
From studylib.net
Lab 33 calorimetry lab Hypothesis Calorimetry Lab Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Find the heat capacity (cp) of a calorimeter and contents (calibration). this experiment has three primary objectives: calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Use hess’s. Hypothesis Calorimetry Lab.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Hypothesis Calorimetry Lab in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. the heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. this experiment has three primary objectives: Specific heat is an intensive property of. Hypothesis Calorimetry Lab.
From www.studypool.com
SOLUTION Calorimetry lab gizmo all answers correct Studypool Hypothesis Calorimetry Lab calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. to measure thermochemical properties of aqueous reactions and substances using a simple calorimeter.. Hypothesis Calorimetry Lab.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Calorimeter Studypool Hypothesis Calorimetry Lab Use hess’s law to determine. Determine the hrxn, the enthalpy of. the heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. show how. Hypothesis Calorimetry Lab.
From fyoislawz.blob.core.windows.net
Calorimetry Online Experiment at Catherine blog Hypothesis Calorimetry Lab Use hess’s law to determine. Find the heat capacity (cp) of a calorimeter and contents (calibration). the heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the. this experiment has three primary objectives: Specific heat is an intensive property of a single phase (solid, liquid or gas). Hypothesis Calorimetry Lab.
From kaffee.50webs.com
Lab Calorimetry Hypothesis Calorimetry Lab lab session 9, experiment 8: Find the heat capacity (cp) of a calorimeter and contents (calibration). in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. the heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and. Hypothesis Calorimetry Lab.
From www.studocu.com
Calorimetry Lab Report Physics 212 General Physics With Calculus 212 StuDocu Hypothesis Calorimetry Lab lab session 9, experiment 8: Use hess’s law to determine. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. Find the heat capacity (cp) of a calorimeter and. Hypothesis Calorimetry Lab.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Hypothesis Calorimetry Lab Find the heat capacity (cp) of a calorimeter and contents (calibration). this experiment has three primary objectives: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. show how these equations must be summed together according to hess’s law to determine δh δ h for the combustion of iron (target equation.. Hypothesis Calorimetry Lab.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Hypothesis Calorimetry Lab Find the heat capacity (cp) of a calorimeter and contents (calibration). Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes. this experiment has three primary objectives: lab session 9, experiment 8: the heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter. Hypothesis Calorimetry Lab.