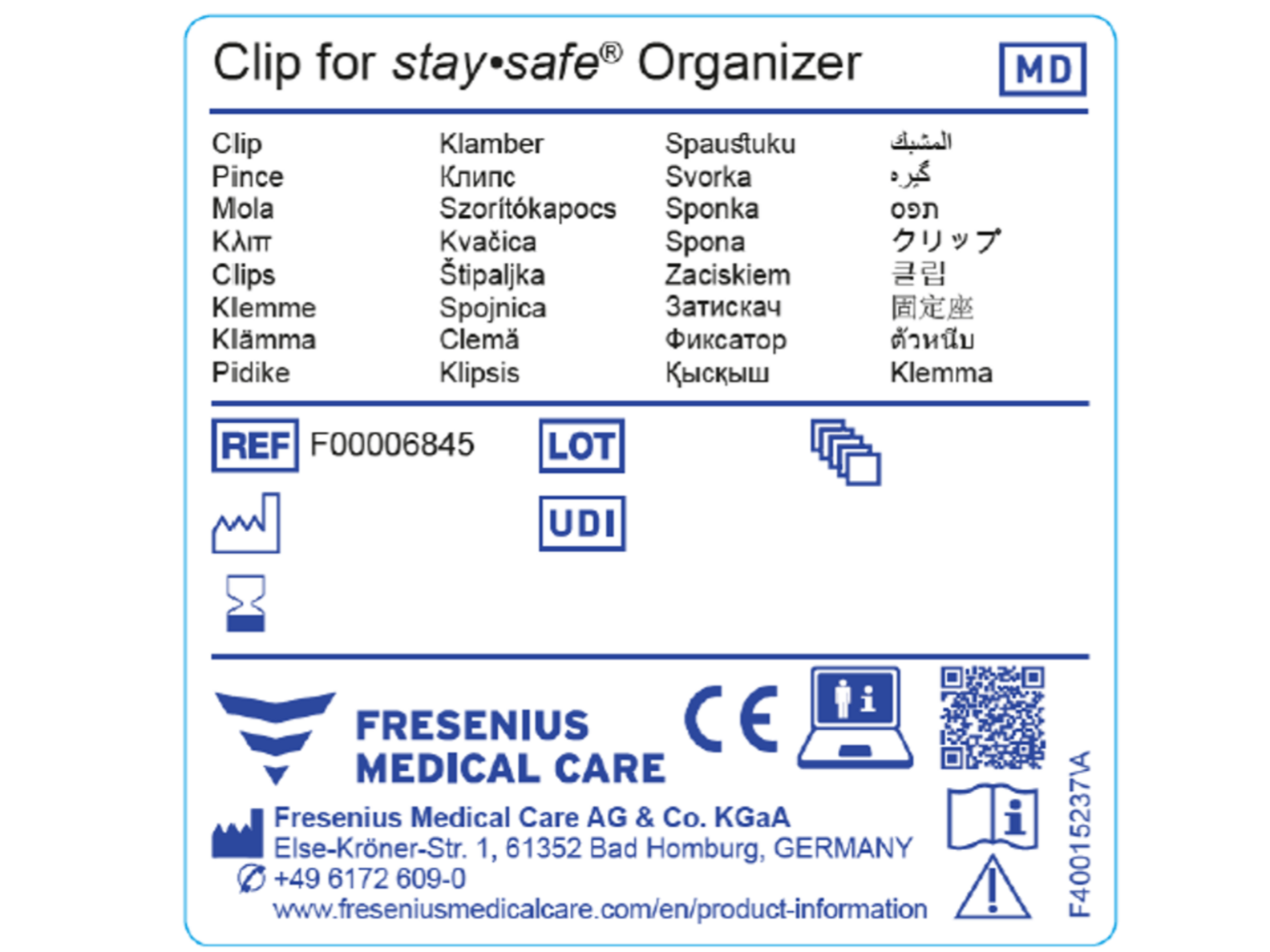
Medical Device Labeling Requirements Europe . On october 14, 2021, the. The eu’s revised medical device regulation went into effect in may 2021. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. updated medical device regulations.
from www.freseniusmedicalcare.com
regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. On october 14, 2021, the. The eu’s revised medical device regulation went into effect in may 2021. updated medical device regulations. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of.
Medical device regulation Fresenius Medical Care
Medical Device Labeling Requirements Europe Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. On october 14, 2021, the. The eu’s revised medical device regulation went into effect in may 2021. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. updated medical device regulations. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Registration & Labeling Medical Device Labeling Requirements Europe Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. The eu’s revised medical device regulation went into effect in may 2021. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. On october 14, 2021, the. the medical devices regulation. Medical Device Labeling Requirements Europe.
From www.mavenrs.uk
Medical Devices Labeling Checklist for EU MDR Compliance Maven Medical Device Labeling Requirements Europe the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. On october 14, 2021, the.. Medical Device Labeling Requirements Europe.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labeling Requirements Europe The eu’s revised medical device regulation went into effect in may 2021. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. updated medical device regulations. medical devices placed in the. Medical Device Labeling Requirements Europe.
From www.freyrsolutions.com
UKCA Marking Requirements for Medical Devices Freyr Global Regulatory Solutions and Services Medical Device Labeling Requirements Europe On october 14, 2021, the. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. The eu’s revised medical device regulation went into effect in may 2021. updated medical device regulations.. Medical Device Labeling Requirements Europe.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements Europe The eu’s revised medical device regulation went into effect in may 2021. updated medical device regulations. On october 14, 2021, the. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. medical devices placed. Medical Device Labeling Requirements Europe.
From medenvoyglobal.com
Medical Device Labeling Requirements in Europe MedEnvoy Medical Device Labeling Requirements Europe Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. On october 14, 2021, the. The eu’s revised medical device regulation went into effect in may 2021. updated medical device regulations. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. medical devices placed. Medical Device Labeling Requirements Europe.
From kukrejahospital.com
MDR Labelling Requirements in Europe for Medical Devices Medical Device Labeling Requirements Europe regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The eu’s revised medical device regulation went into effect in may 2021. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. On october 14, 2021, the. the medical devices regulation 2017/745/eu. Medical Device Labeling Requirements Europe.
From www.meddeviceonline.com
Infographic Medical Device Label Before And After EU MDR 10 Sticking Points Medical Device Labeling Requirements Europe regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. On october 14, 2021, the. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask. Medical Device Labeling Requirements Europe.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements Europe the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. updated medical device. Medical Device Labeling Requirements Europe.
From www.tailoredlabel.com
Medical Device Regulation The Impact on Medical Device Labeling TLP Medical Device Labeling Requirements Europe On october 14, 2021, the. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The eu’s revised medical device regulation went into effect in may 2021. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. updated medical device. Medical Device Labeling Requirements Europe.
From www.presentationeze.com
FDA Medical Device Labeling.PresentationEZE Medical Device Labeling Requirements Europe updated medical device regulations. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. Every manufacturer is required to incorporate the medical device symbol, which states. Medical Device Labeling Requirements Europe.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download ID3400285 Medical Device Labeling Requirements Europe updated medical device regulations. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. The eu’s revised medical device regulation went into effect in may 2021. the medical devices regulation. Medical Device Labeling Requirements Europe.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Labeling Requirements Europe regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. updated medical device regulations. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity. Medical Device Labeling Requirements Europe.
From platohealth.ai
Ultimate Guide To Device Class Requirements Under EU MDR PlatoHealth Medical Device Labeling Requirements Europe medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. The eu’s revised medical device regulation went into effect in may 2021. On october 14, 2021, the. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive.. Medical Device Labeling Requirements Europe.
From old.sermitsiaq.ag
Medical Device Label Template Medical Device Labeling Requirements Europe The eu’s revised medical device regulation went into effect in may 2021. updated medical device regulations. On october 14, 2021, the. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information. Medical Device Labeling Requirements Europe.
From fyofscoog.blob.core.windows.net
In Vitro Diagnostic Device Labeling Requirements at Randi Coleman blog Medical Device Labeling Requirements Europe Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. regulation (eu) 2017/745. Medical Device Labeling Requirements Europe.
From www.ce-marking.com
Guide on Class IIb MDD Medical Devices CE marking (mark) & European (EU) Authorized Medical Device Labeling Requirements Europe The eu’s revised medical device regulation went into effect in may 2021. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. medical devices placed in the eu market must be labelled with the ce. Medical Device Labeling Requirements Europe.
From easymedicaldevice.com
How to Create a Label as per EU MDR 2017/745? Medical Device Labeling Requirements Europe On october 14, 2021, the. The eu’s revised medical device regulation went into effect in may 2021. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. updated medical device regulations. regulation (eu) 2017/745. Medical Device Labeling Requirements Europe.
From www.microscan.com
Label Compliance and the New European Medical Device Regulations Medical Device Labeling Requirements Europe regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. On. Medical Device Labeling Requirements Europe.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labeling Requirements Europe On october 14, 2021, the. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. The eu’s revised medical device regulation went into effect in may 2021. updated medical device regulations. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information. Medical Device Labeling Requirements Europe.
From blog.clevercompliance.io
EU Medical Device Labelling Requirements Clever Compliance Medical Device Labeling Requirements Europe regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. updated medical device regulations. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity. Medical Device Labeling Requirements Europe.
From mdlaw.eu
MDR Checklist Labelling & IFU Requirements · MDlaw Information platform on European medical Medical Device Labeling Requirements Europe regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. . Medical Device Labeling Requirements Europe.
From www.orielstat.com
Understanding FDA and EU Medical Device Labeling Requirements Oriel STAT A MATRIX Blog Medical Device Labeling Requirements Europe regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. The eu’s revised medical device regulation went into effect in may 2021. On october 14, 2021, the.. Medical Device Labeling Requirements Europe.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Device Labeling Requirements Europe On october 14, 2021, the. updated medical device regulations. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. regulation (eu) 2017/745 of the european parliament and of the council. Medical Device Labeling Requirements Europe.
From dokumen.tips
(PDF) EU Medical Device Labeling DOKUMEN.TIPS Medical Device Labeling Requirements Europe The eu’s revised medical device regulation went into effect in may 2021. On october 14, 2021, the. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. medical devices placed in the eu market must. Medical Device Labeling Requirements Europe.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labeling Requirements Europe Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. The eu’s revised medical device regulation went into effect in may 2021. updated medical device regulations. medical devices placed in the eu market must. Medical Device Labeling Requirements Europe.
From www.techsollifesciences.com
EU MDR & IVDR Medical Device Labelling Requirements Medical Device Labeling Requirements Europe the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. On october 14, 2021, the. The eu’s revised medical device regulation went into effect in may 2021. medical devices placed. Medical Device Labeling Requirements Europe.
From lifesciences.csoftintl.com
The EU MDR Labeling Journey Best Practices for Navigating the Latest Medical Device Labeling Medical Device Labeling Requirements Europe On october 14, 2021, the. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. updated medical device regulations. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. medical devices placed in the eu market must be labelled with the. Medical Device Labeling Requirements Europe.
From gbu-taganskij.ru
EU MDR 2017/745 Medical Device Labeling Compliance, 48 OFF Medical Device Labeling Requirements Europe Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. On october 14, 2021, the. The eu’s revised medical device regulation went into effect in may 2021. the medical devices regulation 2017/745/eu. Medical Device Labeling Requirements Europe.
From www.orielstat.com
Class 1 Medical Device Requirements Oriel STAT A MATRIX Medical Device Labeling Requirements Europe Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. The eu’s revised medical device regulation went into effect in may 2021. On october 14, 2021, the. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. regulation (eu) 2017/745 of. Medical Device Labeling Requirements Europe.
From www.freseniusmedicalcare.com
Medical device regulation Fresenius Medical Care Medical Device Labeling Requirements Europe the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. On october 14, 2021, the. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. The eu’s revised medical device regulation went into effect in may 2021. regulation (eu) 2017/745 of the european parliament and. Medical Device Labeling Requirements Europe.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements Europe Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. updated medical device regulations. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information. Medical Device Labeling Requirements Europe.
From medenvoyglobal.com
Medical Device Labeling Requirements in Europe MedEnvoy Medical Device Labeling Requirements Europe Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. . Medical Device Labeling Requirements Europe.
From medicaldevicelicense.com
EU MDR Medical Device Labeling RequirementsA Complete Guide Medical Device Labeling Requirements Europe the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. medical devices placed in the eu market must be labelled with the ce marking to communicate conformity with the requirements of. Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. The eu’s revised medical. Medical Device Labeling Requirements Europe.
From www.opal-labelmanagement.com
White paper Medical Device Labeling Requirements EU 🩺 Medical Device Labeling Requirements Europe Every manufacturer is required to incorporate the medical device symbol, which states that the product supplied. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical devices regulation 2017/745/eu (‘mdr’) has new requirements that ask for various kinds of information to. The eu’s revised medical device. Medical Device Labeling Requirements Europe.