Bromine Has How Many Neutrons . In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. Bromine has 35 protons and electrons in its structure. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. It has an atomic weight of 79.904 and a mass. The number of neutrons depends on the isotope of the element. These blocks are named for the characteristic spectra. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Elements are organised into blocks by the orbital type in which the outer electrons are found. The total number of neutrons in the nucleus of an atom is called the neutron number. These consist of an atomic nucleus with 35 protons. The bromine atom has two stable isotopes. All atomic nuclei of the chemical element bromine are summarized under bromine isotopes;
from periodictableguide.com
The bromine atom has two stable isotopes. All atomic nuclei of the chemical element bromine are summarized under bromine isotopes; It has an atomic weight of 79.904 and a mass. These consist of an atomic nucleus with 35 protons. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The number of neutrons depends on the isotope of the element. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. Elements are organised into blocks by the orbital type in which the outer electrons are found. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Bromine has 35 protons and electrons in its structure.
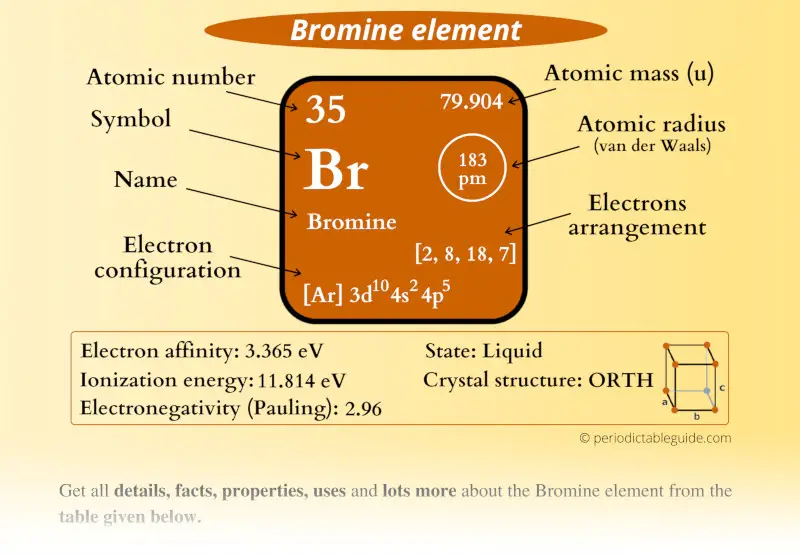
Bromine (Br) Periodic Table (Element Information & More)
Bromine Has How Many Neutrons It has an atomic weight of 79.904 and a mass. These consist of an atomic nucleus with 35 protons. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. The total number of neutrons in the nucleus of an atom is called the neutron number. It has an atomic weight of 79.904 and a mass. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Elements are organised into blocks by the orbital type in which the outer electrons are found. Bromine has 35 protons and electrons in its structure. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The number of neutrons depends on the isotope of the element. These blocks are named for the characteristic spectra. The bromine atom has two stable isotopes. All atomic nuclei of the chemical element bromine are summarized under bromine isotopes;
From www.gauthmath.com
Solved a, supratomt par tcre 9. How many protons, electron, and Bromine Has How Many Neutrons The number of neutrons depends on the isotope of the element. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. All atomic nuclei of the chemical element bromine are summarized under bromine isotopes; These blocks are named for the characteristic spectra. Bromine is the 35th element in. Bromine Has How Many Neutrons.
From app.emaze.com
Bromine ) on emaze Bromine Has How Many Neutrons Elements are organised into blocks by the orbital type in which the outer electrons are found. All atomic nuclei of the chemical element bromine are summarized under bromine isotopes; Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. The total number of neutrons in the nucleus of an atom. Bromine Has How Many Neutrons.
From www.sliderbase.com
The Halogens Presentation Chemistry Bromine Has How Many Neutrons The bromine atom has two stable isotopes. It has an atomic weight of 79.904 and a mass. The number of neutrons depends on the isotope of the element. The total number of neutrons in the nucleus of an atom is called the neutron number. These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital. Bromine Has How Many Neutrons.
From material-properties.org
Bromine Periodic Table and Atomic Properties Bromine Has How Many Neutrons In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. The bromine atom has two stable isotopes. All atomic nuclei of the chemical element bromine are summarized under bromine isotopes; Elements are organised into blocks by the orbital type in which the outer electrons are found. The number of neutrons depends. Bromine Has How Many Neutrons.
From www.nuclear-power.com
Bromine Atomic Number Atomic Mass Density of Bromine nuclear Bromine Has How Many Neutrons These consist of an atomic nucleus with 35 protons. It has an atomic weight of 79.904 and a mass. Elements are organised into blocks by the orbital type in which the outer electrons are found. The bromine atom has two stable isotopes. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number. Bromine Has How Many Neutrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron Bromine Has How Many Neutrons These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found. It has an atomic weight of 79.904 and a mass. Bromine has 35 protons and electrons in its structure. These consist of an atomic nucleus with 35 protons. Bromine is the 35th element in the periodic. Bromine Has How Many Neutrons.
From slideplayer.com
Chapter 5 Atomic Structure and The Periodic Table. ppt download Bromine Has How Many Neutrons Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Bromine has 35 protons and electrons in its structure. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Elements are organised into blocks by the orbital type. Bromine Has How Many Neutrons.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Has How Many Neutrons Bromine has 35 protons and electrons in its structure. The total number of neutrons in the nucleus of an atom is called the neutron number. The number of neutrons depends on the isotope of the element. Elements are organised into blocks by the orbital type in which the outer electrons are found. The bromine atom has two stable isotopes. It. Bromine Has How Many Neutrons.
From www.numerade.com
SOLVED 'A bromine atom has an atomic number of 35 and an atomic mass Bromine Has How Many Neutrons Bromine has 35 protons and electrons in its structure. The total number of neutrons in the nucleus of an atom is called the neutron number. It has an atomic weight of 79.904 and a mass. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. All atomic nuclei of the chemical. Bromine Has How Many Neutrons.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Has How Many Neutrons Elements are organised into blocks by the orbital type in which the outer electrons are found. These consist of an atomic nucleus with 35 protons. It has an atomic weight of 79.904 and a mass. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. These blocks are named for the. Bromine Has How Many Neutrons.
From www.slideshare.net
Atomic concepts Bromine Has How Many Neutrons Bromine has 35 protons and electrons in its structure. Elements are organised into blocks by the orbital type in which the outer electrons are found. These consist of an atomic nucleus with 35 protons. It has an atomic weight of 79.904 and a mass. All atomic nuclei of the chemical element bromine are summarized under bromine isotopes; Bromine is the. Bromine Has How Many Neutrons.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Has How Many Neutrons Bromine has 35 protons and electrons in its structure. These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found. The number of neutrons depends on the isotope of the element. The bromine atom has two stable isotopes. In this video we’ll use the periodic table and. Bromine Has How Many Neutrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition and electron Bromine Has How Many Neutrons The total number of neutrons in the nucleus of an atom is called the neutron number. It has an atomic weight of 79.904 and a mass. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. These consist of an atomic nucleus with 35 protons. All atomic nuclei. Bromine Has How Many Neutrons.
From putney-lettings.blogspot.com
Bromine Periodic Table Protons Neutrons Electrons All About Image HD Bromine Has How Many Neutrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. It has an atomic weight of 79.904 and a mass. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. All atomic nuclei of the chemical element bromine. Bromine Has How Many Neutrons.
From www.alamy.com
Symbol and electron diagram for Bromine Stock Vector Image & Art Alamy Bromine Has How Many Neutrons The total number of neutrons in the nucleus of an atom is called the neutron number. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. All atomic nuclei of the. Bromine Has How Many Neutrons.
From www.gauthmath.com
Solved How many protons, electron and neutron does bromine ion have Bromine Has How Many Neutrons Bromine has 35 protons and electrons in its structure. The total number of neutrons in the nucleus of an atom is called the neutron number. These consist of an atomic nucleus with 35 protons. Elements are organised into blocks by the orbital type in which the outer electrons are found. It has an atomic weight of 79.904 and a mass.. Bromine Has How Many Neutrons.
From www.slideshare.net
Bromine berry Bromine Has How Many Neutrons All atomic nuclei of the chemical element bromine are summarized under bromine isotopes; The total number of neutrons in the nucleus of an atom is called the neutron number. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. These blocks are named for the characteristic spectra. These. Bromine Has How Many Neutrons.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Bromine Has How Many Neutrons All atomic nuclei of the chemical element bromine are summarized under bromine isotopes; Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found. It has an. Bromine Has How Many Neutrons.
From www.alamy.com
Bromine atom, with mass and energy levels. Vector illustration Stock Bromine Has How Many Neutrons In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. Bromine has 35 protons and electrons in its structure. Elements are organised into blocks by the orbital type in which the outer electrons are found. The number of neutrons depends on the isotope of the element. Bromine is the 35th element. Bromine Has How Many Neutrons.
From www.slideserve.com
PPT Head a paper for class notes Record all drawings and data charts Bromine Has How Many Neutrons These blocks are named for the characteristic spectra. These consist of an atomic nucleus with 35 protons. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. It has an atomic weight of 79.904 and a mass. The total number of neutrons in the nucleus of an atom is called the. Bromine Has How Many Neutrons.
From www.youtube.com
Consider bromine79. Select the lists with the correct number of Bromine Has How Many Neutrons These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found. Bromine has 35 protons and electrons in its structure. All atomic nuclei of the chemical element bromine are summarized under bromine isotopes; These consist of an atomic nucleus with 35 protons. The bromine atom has two. Bromine Has How Many Neutrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Bromine (Br Bromine Has How Many Neutrons Bromine has 35 protons and electrons in its structure. It has an atomic weight of 79.904 and a mass. The number of neutrons depends on the isotope of the element. Elements are organised into blocks by the orbital type in which the outer electrons are found. In this video we’ll use the periodic table and a few simple rules to. Bromine Has How Many Neutrons.
From www.numerade.com
SOLVED Determine the number of neutrons in a bromine nucleus, which Bromine Has How Many Neutrons All atomic nuclei of the chemical element bromine are summarized under bromine isotopes; These blocks are named for the characteristic spectra. The total number of neutrons in the nucleus of an atom is called the neutron number. In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. These consist of an. Bromine Has How Many Neutrons.
From www.schoolmykids.com
Bromine (Br) Element Information, Facts, Properties, Uses Periodic Bromine Has How Many Neutrons The total number of neutrons in the nucleus of an atom is called the neutron number. The number of neutrons depends on the isotope of the element. These consist of an atomic nucleus with 35 protons. Elements are organised into blocks by the orbital type in which the outer electrons are found. It has an atomic weight of 79.904 and. Bromine Has How Many Neutrons.
From www.slideserve.com
PPT Topic Chemistry Aim Explain how elements are classified in Bromine Has How Many Neutrons The bromine atom has two stable isotopes. The total number of neutrons in the nucleus of an atom is called the neutron number. These consist of an atomic nucleus with 35 protons. It has an atomic weight of 79.904 and a mass. Elements are organised into blocks by the orbital type in which the outer electrons are found. Bromine is. Bromine Has How Many Neutrons.
From material-properties.org
Bromine Protons Neutrons Electrons Electron Configuration Bromine Has How Many Neutrons These consist of an atomic nucleus with 35 protons. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Bromine has 35 protons and electrons in its structure. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell.. Bromine Has How Many Neutrons.
From www.dreamstime.com
Bromine stock illustration. Illustration of render, formula 139651101 Bromine Has How Many Neutrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Elements are organised into blocks by the orbital type in which the outer electrons are found. Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. In this. Bromine Has How Many Neutrons.
From periodictableguide.com
Bromine (Br) Periodic Table (Element Information & More) Bromine Has How Many Neutrons Bromine is the 35th element in the periodic table and has a symbol of br and atomic number of 35. Elements are organised into blocks by the orbital type in which the outer electrons are found. These consist of an atomic nucleus with 35 protons. All atomic nuclei of the chemical element bromine are summarized under bromine isotopes; In this. Bromine Has How Many Neutrons.
From sciencenotes.org
Bromine Facts Atomic Number 35 and Element Symbol Br Bromine Has How Many Neutrons Elements are organised into blocks by the orbital type in which the outer electrons are found. The bromine atom has two stable isotopes. All atomic nuclei of the chemical element bromine are summarized under bromine isotopes; These consist of an atomic nucleus with 35 protons. Bromine has 35 protons and electrons in its structure. The total number of neutrons in. Bromine Has How Many Neutrons.
From www.alamy.com
Bromine (Br). Diagram of the nuclear composition, electron Bromine Has How Many Neutrons These blocks are named for the characteristic spectra. It has an atomic weight of 79.904 and a mass. The number of neutrons depends on the isotope of the element. Elements are organised into blocks by the orbital type in which the outer electrons are found. These consist of an atomic nucleus with 35 protons. Bromine has 35 protons and electrons. Bromine Has How Many Neutrons.
From slideplayer.com
Isotopes and Ions Section 33 Continued. ppt download Bromine Has How Many Neutrons Bromine has 35 protons and electrons in its structure. These blocks are named for the characteristic spectra. Elements are organised into blocks by the orbital type in which the outer electrons are found. It has an atomic weight of 79.904 and a mass. In this video we’ll use the periodic table and a few simple rules to find the protons,. Bromine Has How Many Neutrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Has How Many Neutrons In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. All atomic nuclei of the chemical element bromine are summarized under bromine isotopes; The total number of neutrons in the nucleus of an atom is called the neutron number. These consist of an atomic nucleus with 35 protons. The bromine atom. Bromine Has How Many Neutrons.
From chemistry.about.com
Atoms Diagrams Electron Configurations of Elements Bromine Has How Many Neutrons In this video we’ll use the periodic table and a few simple rules to find the protons, electrons, and. The number of neutrons depends on the isotope of the element. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Bromine has 35 protons and electrons in its. Bromine Has How Many Neutrons.
From ar.inspiredpencil.com
Atomic Structure Of Bromine Bromine Has How Many Neutrons These blocks are named for the characteristic spectra. The bromine atom has two stable isotopes. All atomic nuclei of the chemical element bromine are summarized under bromine isotopes; 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Bromine has 35 protons and electrons in its structure. In. Bromine Has How Many Neutrons.
From material-properties.org
Bromine Protons Neutrons Electrons Electron Configuration Bromine Has How Many Neutrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. The number of neutrons depends on the isotope of the element. These consist of an atomic nucleus with 35 protons. All atomic nuclei of the chemical element bromine are summarized under bromine isotopes; The bromine atom has two. Bromine Has How Many Neutrons.