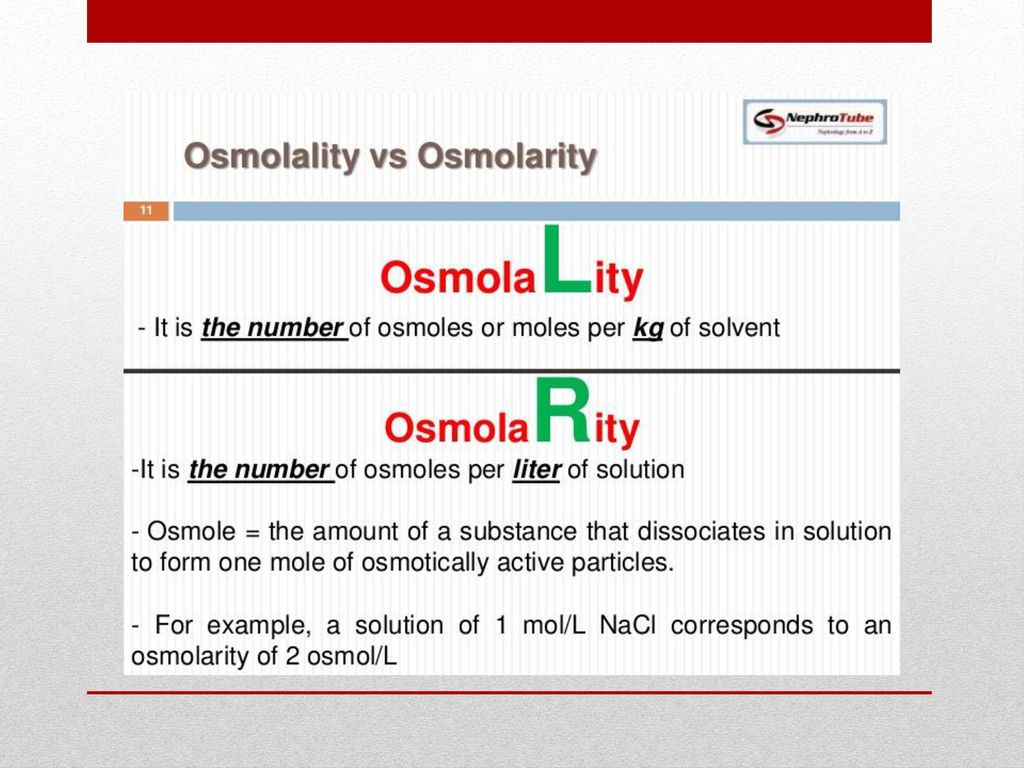
Osmolality Definition Physiology . Define osmosis and explain its role within molecules; The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. In biologic systems, osmolality is expressed as mosm/kg. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Explain why osmoregulation and osmotic balance are important body functions;. Osmolality is the concentration of osmoles in a mass of solvent. The term osmolality expresses concentrations. Osmolarity of a solution is the number of osmoles of solute per. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. In terms of physiological fluids, particularly plasma, osmolality refers to the concentration of dissolved electrolytes such as sodium,.
from slideplayer.com
Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. Osmolarity of a solution is the number of osmoles of solute per. Osmolality is the concentration of osmoles in a mass of solvent. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Define osmosis and explain its role within molecules; The term osmolality expresses concentrations. In biologic systems, osmolality is expressed as mosm/kg. Explain why osmoregulation and osmotic balance are important body functions;. In terms of physiological fluids, particularly plasma, osmolality refers to the concentration of dissolved electrolytes such as sodium,.
Fluid and electrolyte balance ppt download
Osmolality Definition Physiology In biologic systems, osmolality is expressed as mosm/kg. Osmolality is the concentration of osmoles in a mass of solvent. In terms of physiological fluids, particularly plasma, osmolality refers to the concentration of dissolved electrolytes such as sodium,. The term osmolality expresses concentrations. Define osmosis and explain its role within molecules; Osmolarity of a solution is the number of osmoles of solute per. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. In biologic systems, osmolality is expressed as mosm/kg. Explain why osmoregulation and osmotic balance are important body functions;. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution.
From www.pinterest.com
the word osmoliaity is written in two different languages on a blue Osmolality Definition Physiology In biologic systems, osmolality is expressed as mosm/kg. Define osmosis and explain its role within molecules; Osmolality is the concentration of osmoles in a mass of solvent. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. Osmolarity of a solution is the number of osmoles of solute per. Explain why osmoregulation and osmotic balance. Osmolality Definition Physiology.
From www.vrogue.co
Blood Osmolality Test Definition And Patient Educatio vrogue.co Osmolality Definition Physiology Define osmosis and explain its role within molecules; The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. Osmolality is the concentration of osmoles in a mass of solvent. Osmolarity of a solution is the number of osmoles of. Osmolality Definition Physiology.
From www.slideshare.net
Regulation of osmolarity Osmolality Definition Physiology Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. The term osmolality expresses concentrations. In terms of physiological fluids, particularly. Osmolality Definition Physiology.
From study.com
Serum Osmolality Definition, Calculation & Interpretation Lesson Osmolality Definition Physiology Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. In terms of physiological fluids, particularly plasma, osmolality refers to the concentration of dissolved electrolytes such as sodium,. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. The standard definition of tonicity usually incorporates some mention. Osmolality Definition Physiology.
From www.researchgate.net
Osmoreceptors in the hypothalamus detect increased serum osmolality Osmolality Definition Physiology The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Osmolarity of a solution is the number of osmoles of solute per. Osmolality is the concentration of osmoles in a mass of solvent. In terms of. Osmolality Definition Physiology.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6798349 Osmolality Definition Physiology In biologic systems, osmolality is expressed as mosm/kg. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Define osmosis and explain its role within molecules; Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. The standard definition of tonicity usually incorporates some mention of osmotic. Osmolality Definition Physiology.
From ar.inspiredpencil.com
Osmolality Osmolality Definition Physiology Explain why osmoregulation and osmotic balance are important body functions;. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. In terms of physiological fluids, particularly plasma, osmolality refers to the concentration of dissolved electrolytes such as sodium,. Osmolarity. Osmolality Definition Physiology.
From www.slideshare.net
L&E Chapter 003 Lo Osmolality Definition Physiology Define osmosis and explain its role within molecules; The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Explain why osmoregulation and osmotic balance are important body functions;. Osmolality is the concentration of osmoles in a. Osmolality Definition Physiology.
From drawittoknowit.com
Anatomy & Physiology Fundamentals Osmosis and Osmolarity ditki Osmolality Definition Physiology Osmolality is the concentration of osmoles in a mass of solvent. In biologic systems, osmolality is expressed as mosm/kg. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. In terms of physiological fluids, particularly plasma,. Osmolality Definition Physiology.
From www.youtube.com
Osmolality vs Osmolarity (with a mnemonic) Physiology and Chemistry Osmolality Definition Physiology Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. The term osmolality expresses concentrations. Explain why osmoregulation and osmotic balance are important body functions;. Osmolarity of a solution is the number of osmoles of solute per. In biologic systems, osmolality is expressed as mosm/kg. In terms of physiological fluids, particularly plasma,. Osmolality Definition Physiology.
From doctorlib.info
The Cell Membrane Physiology An Illustrated Review Osmolality Definition Physiology Explain why osmoregulation and osmotic balance are important body functions;. In biologic systems, osmolality is expressed as mosm/kg. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. Osmolarity of a solution is the number of osmoles. Osmolality Definition Physiology.
From www.slideserve.com
PPT ENTERAL NUTRITION PowerPoint Presentation ID225635 Osmolality Definition Physiology Osmolality is the concentration of osmoles in a mass of solvent. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. Define osmosis and explain its role within molecules; Osmolarity of a solution is the number of osmoles of solute per. Explain why osmoregulation and osmotic balance are important body functions;. The term osmolality. Osmolality Definition Physiology.
From basicmedicalkey.com
Control of Body Fluid Osmolality and Volume Basicmedical Key Osmolality Definition Physiology Explain why osmoregulation and osmotic balance are important body functions;. Osmolarity of a solution is the number of osmoles of solute per. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. The term osmolality expresses concentrations. In biologic systems, osmolality is expressed as mosm/kg. Define osmosis and explain its role within molecules; In terms. Osmolality Definition Physiology.
From study.com
Osmolarity Definition, Formula & Calculations Video & Lesson Osmolality Definition Physiology Osmolarity of a solution is the number of osmoles of solute per. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. In biologic systems, osmolality is expressed as mosm/kg. In terms of physiological fluids, particularly plasma, osmolality refers to the concentration of dissolved electrolytes such as sodium,. Define osmosis and explain. Osmolality Definition Physiology.
From www.sqadia.com
Regulation of Plasma Osmolality Osmolality Definition Physiology Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Osmolality is the concentration of osmoles in a mass of solvent. Osmolarity of a solution is the number of osmoles of solute per. In terms of physiological fluids, particularly plasma, osmolality refers to the concentration of dissolved electrolytes such as sodium,. The. Osmolality Definition Physiology.
From www.pinterest.com
Difference Between Osmolarity and Osmolality Definition, Explanation Osmolality Definition Physiology The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. Osmolality is the concentration of osmoles in a mass of solvent. In terms of physiological fluids, particularly plasma, osmolality refers to the concentration of dissolved electrolytes such as sodium,. Osmolarity of a solution is the number of osmoles of solute per. Explain why osmoregulation. Osmolality Definition Physiology.
From askanydifference.com
Osmolality vs Osmolarity Difference and Comparison Osmolality Definition Physiology Osmolarity of a solution is the number of osmoles of solute per. In terms of physiological fluids, particularly plasma, osmolality refers to the concentration of dissolved electrolytes such as sodium,. In biologic systems, osmolality is expressed as mosm/kg. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Osmolality is the concentration. Osmolality Definition Physiology.
From slideplayer.com
Fluid and electrolyte balance ppt download Osmolality Definition Physiology Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. Explain why osmoregulation and osmotic balance are important body functions;. Osmolality is the concentration of osmoles in a mass of solvent. In terms of physiological fluids, particularly plasma, osmolality refers to the concentration of dissolved electrolytes such as sodium,. The standard definition of tonicity usually. Osmolality Definition Physiology.
From www.youtube.com
Chemistry Basics Osmolarity, Osmolality and Tonicity YouTube Osmolality Definition Physiology The term osmolality expresses concentrations. In biologic systems, osmolality is expressed as mosm/kg. Define osmosis and explain its role within molecules; The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Osmolality is the concentration of. Osmolality Definition Physiology.
From lookfordiagnosis.com
Osmolar Concentration; Ionic Strength; Osmolarity; Osmolality Osmolality Definition Physiology The term osmolality expresses concentrations. Osmolality is the concentration of osmoles in a mass of solvent. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Explain why osmoregulation and osmotic balance are important body functions;. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between.. Osmolality Definition Physiology.
From www.slideshare.net
Formula osmolality and nutritional needs Osmolality Definition Physiology Osmolarity of a solution is the number of osmoles of solute per. The term osmolality expresses concentrations. Define osmosis and explain its role within molecules; The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. In terms of physiological fluids, particularly plasma, osmolality refers to the concentration of dissolved electrolytes such as sodium,. In. Osmolality Definition Physiology.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 1. Osmosis ditki medical Osmolality Definition Physiology Osmolality is the concentration of osmoles in a mass of solvent. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. The term osmolality expresses concentrations. In terms of physiological fluids, particularly plasma, osmolality refers to the. Osmolality Definition Physiology.
From www.slideserve.com
PPT Tonicity, Osmoticity , Osmolarity , & Osmolality PowerPoint Osmolality Definition Physiology Osmolality is the concentration of osmoles in a mass of solvent. In biologic systems, osmolality is expressed as mosm/kg. Define osmosis and explain its role within molecules; The term osmolality expresses concentrations. Explain why osmoregulation and osmotic balance are important body functions;. Osmolarity of a solution is the number of osmoles of solute per. Osmolality of a solution is the. Osmolality Definition Physiology.
From pediaa.com
Difference Between Osmolarity and Tonicity Osmolality Definition Physiology Explain why osmoregulation and osmotic balance are important body functions;. The term osmolality expresses concentrations. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. Osmolality is the concentration of osmoles in a mass of solvent. Osmolarity of a solution is the number of osmoles of solute per. Osmolality is a colligative property of solutions. Osmolality Definition Physiology.
From biologydictionary.net
Osmolarity The Definitive Guide Biology Dictionary Osmolality Definition Physiology The term osmolality expresses concentrations. In terms of physiological fluids, particularly plasma, osmolality refers to the concentration of dissolved electrolytes such as sodium,. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. In biologic systems, osmolality is expressed as mosm/kg. The standard definition of tonicity usually incorporates some mention of osmotic. Osmolality Definition Physiology.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6798349 Osmolality Definition Physiology Define osmosis and explain its role within molecules; Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. Osmolality is the concentration of osmoles in a mass of solvent. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Osmolarity of a solution is the number of. Osmolality Definition Physiology.
From www.pinterest.com
Osmolality Physiology, Vascular, Medicine Osmolality Definition Physiology In biologic systems, osmolality is expressed as mosm/kg. Define osmosis and explain its role within molecules; Osmolality is the concentration of osmoles in a mass of solvent. Explain why osmoregulation and osmotic balance are important body functions;. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. The term osmolality expresses concentrations. The standard definition. Osmolality Definition Physiology.
From www.slideserve.com
PPT Fluids and Electrolytes PowerPoint Presentation, free download Osmolality Definition Physiology The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. In terms of physiological fluids, particularly plasma, osmolality refers to the concentration of dissolved electrolytes such as sodium,. Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. The term osmolality expresses concentrations. Explain why osmoregulation. Osmolality Definition Physiology.
From www.youtube.com
Using VolumeOsmolality Diagrams to Understand Body Fluid Status YouTube Osmolality Definition Physiology Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. Osmolality is the concentration of osmoles in a mass of solvent. Define osmosis and explain its role within molecules; In biologic systems, osmolality is expressed as. Osmolality Definition Physiology.
From www.scribd.com
Water Balance and Regulation of Osmolality Physiology Urinary System Osmolality Definition Physiology Define osmosis and explain its role within molecules; Explain why osmoregulation and osmotic balance are important body functions;. Osmolarity of a solution is the number of osmoles of solute per. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. Osmolality is the concentration of osmoles in a mass of solvent. The term osmolality expresses. Osmolality Definition Physiology.
From slideplayer.com
Fluid Balance. ppt download Osmolality Definition Physiology Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. The standard definition of tonicity usually incorporates some mention of osmotic pressure or osmolality difference between. The term osmolality expresses concentrations. In biologic systems, osmolality is expressed as mosm/kg. Osmolarity of a solution is the number of osmoles of solute per. Osmolality. Osmolality Definition Physiology.
From www.slideserve.com
PPT TONICITY, OSMOTICITY, OSMOLALITY & OSMOLARITY PowerPoint Osmolality Definition Physiology Define osmosis and explain its role within molecules; Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. In terms of physiological fluids, particularly plasma, osmolality refers to the concentration of dissolved electrolytes such as sodium,. Osmolality is the concentration of osmoles in a mass of solvent. The standard definition of tonicity. Osmolality Definition Physiology.
From ditki.com
Biochemistry Glossary Osmosis & Osmolarity 2. Osmolarity ditki Osmolality Definition Physiology Osmolarity of a solution is the number of osmoles of solute per. The term osmolality expresses concentrations. Osmolality is the concentration of osmoles in a mass of solvent. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. Define osmosis and explain its role within molecules; The standard definition of tonicity usually incorporates some mention. Osmolality Definition Physiology.
From www.slideserve.com
PPT IV Therapy PowerPoint Presentation, free download ID9628818 Osmolality Definition Physiology Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Osmolality of a solution is the number of osmoles of solute per kilogram of solvent. Explain why osmoregulation and osmotic balance are important body functions;. In terms of physiological fluids, particularly plasma, osmolality refers to the concentration of dissolved electrolytes such as. Osmolality Definition Physiology.
From www.slideserve.com
PPT Diffusion PowerPoint Presentation, free download ID6798349 Osmolality Definition Physiology Osmolality is a colligative property of solutions that depends on the number of dissolved particles in the solution. Osmolality is the concentration of osmoles in a mass of solvent. Osmolarity of a solution is the number of osmoles of solute per. Explain why osmoregulation and osmotic balance are important body functions;. Define osmosis and explain its role within molecules; Osmolality. Osmolality Definition Physiology.