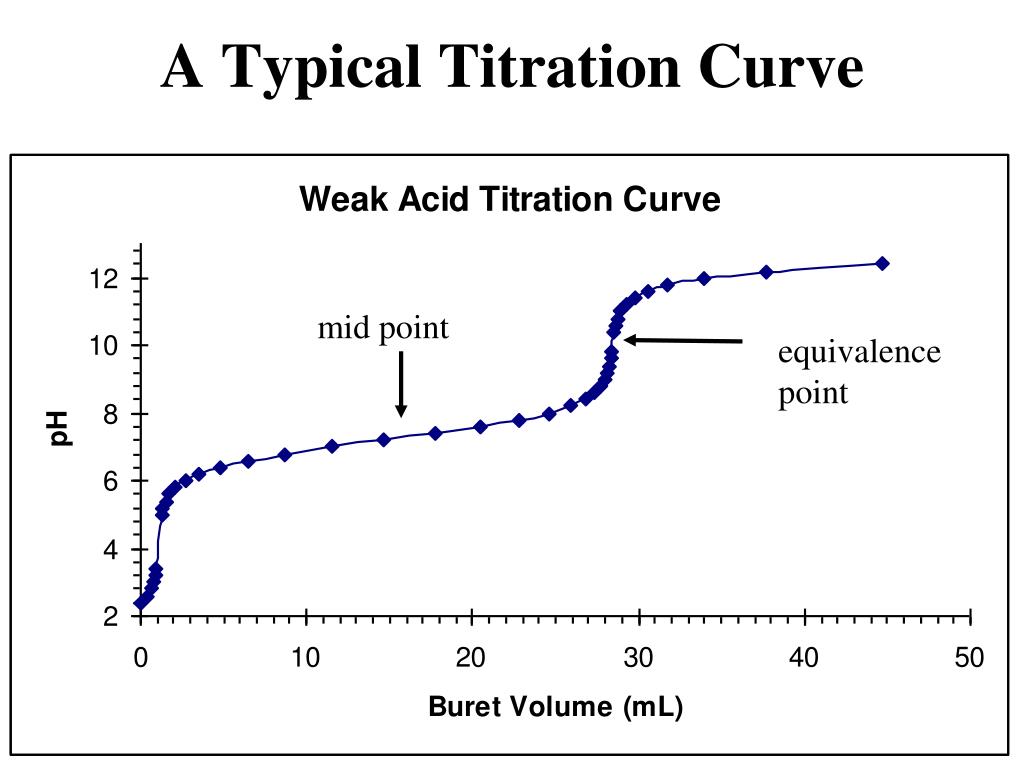
Titration Curve Pi . Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of the carboxyl (2.34) and ammonium (9.60) groups. Undergraduate biochemistry students should have great familiarity with titration curves. If we only add half as much base, only. An essential concept in studying the titration curves of amino acids is the isoelectric point (pi). From the curve we can infer that the $\mathrm{pi}$ is simply the average of the two $\mathrm pk_\mathrm a$ values of the two carboxylic acid groups. These curves allow the prediction of protonation states, charges, and isoelectric points. The isoelectric points range from 5.5 to 6.2. The isoelectric point refers to the ph at which. Titration curves show the neutralization of these acids by added base, and the change in ph.
from www.slideserve.com
The isoelectric point refers to the ph at which. These curves allow the prediction of protonation states, charges, and isoelectric points. An essential concept in studying the titration curves of amino acids is the isoelectric point (pi). How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of the carboxyl (2.34) and ammonium (9.60) groups. Titration curves show the neutralization of these acids by added base, and the change in ph. Undergraduate biochemistry students should have great familiarity with titration curves. If we only add half as much base, only. Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. From the curve we can infer that the $\mathrm{pi}$ is simply the average of the two $\mathrm pk_\mathrm a$ values of the two carboxylic acid groups. The isoelectric points range from 5.5 to 6.2.
PPT How to Interpret Titration Curves PowerPoint Presentation, free
Titration Curve Pi How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of the carboxyl (2.34) and ammonium (9.60) groups. The isoelectric points range from 5.5 to 6.2. Titration curves show the neutralization of these acids by added base, and the change in ph. These curves allow the prediction of protonation states, charges, and isoelectric points. An essential concept in studying the titration curves of amino acids is the isoelectric point (pi). How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of the carboxyl (2.34) and ammonium (9.60) groups. Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. The isoelectric point refers to the ph at which. From the curve we can infer that the $\mathrm{pi}$ is simply the average of the two $\mathrm pk_\mathrm a$ values of the two carboxylic acid groups. Undergraduate biochemistry students should have great familiarity with titration curves. If we only add half as much base, only.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID1205383 Titration Curve Pi Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. These curves allow the prediction of protonation states, charges, and isoelectric points. If we only add half as much base, only. An essential concept in studying the titration curves of amino acids is the isoelectric point (pi). Titration curves show the. Titration Curve Pi.
From mavink.com
Asparagine Titration Curve Titration Curve Pi The isoelectric points range from 5.5 to 6.2. Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. These curves allow the prediction of protonation states, charges, and isoelectric points. If we only add half as much base, only. Titration curves show the neutralization of these acids by added base, and. Titration Curve Pi.
From mungfali.com
Amino Acid Titration Curve Titration Curve Pi These curves allow the prediction of protonation states, charges, and isoelectric points. Undergraduate biochemistry students should have great familiarity with titration curves. If we only add half as much base, only. An essential concept in studying the titration curves of amino acids is the isoelectric point (pi). Titration curves show the neutralization of these acids by added base, and the. Titration Curve Pi.
From mavink.com
Titration Curve Labeled Titration Curve Pi The isoelectric point refers to the ph at which. Undergraduate biochemistry students should have great familiarity with titration curves. An essential concept in studying the titration curves of amino acids is the isoelectric point (pi). Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. Titration curves show the neutralization of. Titration Curve Pi.
From www.vedantu.com
What is the titration curve of glycine? Titration Curve Pi From the curve we can infer that the $\mathrm{pi}$ is simply the average of the two $\mathrm pk_\mathrm a$ values of the two carboxylic acid groups. Titration curves show the neutralization of these acids by added base, and the change in ph. If we only add half as much base, only. Titration curves show the neutralization of these acids by. Titration Curve Pi.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation ID1205383 Titration Curve Pi Titration curves show the neutralization of these acids by added base, and the change in ph. Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. These curves allow the prediction of protonation states, charges, and isoelectric points. How to determine pi from the curve • in diprotic amino acids such. Titration Curve Pi.
From www.youtube.com
Titration curves, pKa, pI, and Buffering regions YouTube Titration Curve Pi The isoelectric points range from 5.5 to 6.2. If we only add half as much base, only. Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. Undergraduate biochemistry students should have great familiarity with titration curves. From the curve we can infer that the $\mathrm{pi}$ is simply the average of. Titration Curve Pi.
From www.reddit.com
in pH titration, how do you determine the (multiple) pKa points from Titration Curve Pi These curves allow the prediction of protonation states, charges, and isoelectric points. An essential concept in studying the titration curves of amino acids is the isoelectric point (pi). How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of the carboxyl (2.34) and ammonium (9.60). Titration Curve Pi.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Curve Pi Undergraduate biochemistry students should have great familiarity with titration curves. The isoelectric points range from 5.5 to 6.2. An essential concept in studying the titration curves of amino acids is the isoelectric point (pi). Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. From the curve we can infer that. Titration Curve Pi.
From mavink.com
Titration Labeled Titration Curve Pi Titration curves show the neutralization of these acids by added base, and the change in ph. Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. From the curve we can infer that the $\mathrm{pi}$ is simply the average of the two $\mathrm pk_\mathrm a$ values of the two carboxylic acid. Titration Curve Pi.
From www.slideshare.net
Chapter 3b Titration Curve Pi An essential concept in studying the titration curves of amino acids is the isoelectric point (pi). The isoelectric point refers to the ph at which. From the curve we can infer that the $\mathrm{pi}$ is simply the average of the two $\mathrm pk_\mathrm a$ values of the two carboxylic acid groups. The isoelectric points range from 5.5 to 6.2. Titration. Titration Curve Pi.
From www.wizeprep.com
Titration Curves Wize University Chemistry Textbook Wizeprep Titration Curve Pi From the curve we can infer that the $\mathrm{pi}$ is simply the average of the two $\mathrm pk_\mathrm a$ values of the two carboxylic acid groups. Titration curves show the neutralization of these acids by added base, and the change in ph. How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi. Titration Curve Pi.
From www.chegg.com
1)According to the titration curve of glycine shown Titration Curve Pi Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. From the curve we can infer that the $\mathrm{pi}$ is simply the average of the two $\mathrm pk_\mathrm a$ values of the two carboxylic acid groups. Undergraduate biochemistry students should have great familiarity with titration curves. The isoelectric points range from. Titration Curve Pi.
From www.youtube.com
Acid Base Titration Curves Simplified YouTube Titration Curve Pi How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of the carboxyl (2.34) and ammonium (9.60) groups. Titration curves show the neutralization of these acids by added base, and the change in ph. These curves allow the prediction of protonation states, charges, and isoelectric. Titration Curve Pi.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve Pi The isoelectric points range from 5.5 to 6.2. The isoelectric point refers to the ph at which. An essential concept in studying the titration curves of amino acids is the isoelectric point (pi). How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of the. Titration Curve Pi.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation, free Titration Curve Pi How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of the carboxyl (2.34) and ammonium (9.60) groups. If we only add half as much base, only. The isoelectric points range from 5.5 to 6.2. Titration curves show the neutralization of these acids by added. Titration Curve Pi.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curve Pi From the curve we can infer that the $\mathrm{pi}$ is simply the average of the two $\mathrm pk_\mathrm a$ values of the two carboxylic acid groups. If we only add half as much base, only. Undergraduate biochemistry students should have great familiarity with titration curves. The isoelectric points range from 5.5 to 6.2. An essential concept in studying the titration. Titration Curve Pi.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve Pi Undergraduate biochemistry students should have great familiarity with titration curves. The isoelectric points range from 5.5 to 6.2. These curves allow the prediction of protonation states, charges, and isoelectric points. If we only add half as much base, only. How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average. Titration Curve Pi.
From fernando-well-key.blogspot.com
How to Describe a Titration Curve Titration Curve Pi Titration curves show the neutralization of these acids by added base, and the change in ph. An essential concept in studying the titration curves of amino acids is the isoelectric point (pi). The isoelectric point refers to the ph at which. These curves allow the prediction of protonation states, charges, and isoelectric points. From the curve we can infer that. Titration Curve Pi.
From www.youtube.com
Titration Curve of Glutamate YouTube Titration Curve Pi Titration curves show the neutralization of these acids by added base, and the change in ph. Undergraduate biochemistry students should have great familiarity with titration curves. These curves allow the prediction of protonation states, charges, and isoelectric points. From the curve we can infer that the $\mathrm{pi}$ is simply the average of the two $\mathrm pk_\mathrm a$ values of the. Titration Curve Pi.
From cwsimons.com
How to Draw Titration Curves of Amino Acids Food Science Toolbox Titration Curve Pi If we only add half as much base, only. These curves allow the prediction of protonation states, charges, and isoelectric points. Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. Undergraduate biochemistry students should have great familiarity with titration curves. Titration curves show the neutralization of these acids by added. Titration Curve Pi.
From www.researchgate.net
Titration curve of BMAA. Titration shows two pK a values, primary amine Titration Curve Pi How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of the carboxyl (2.34) and ammonium (9.60) groups. These curves allow the prediction of protonation states, charges, and isoelectric points. If we only add half as much base, only. Titration curves show the neutralization of. Titration Curve Pi.
From www.researchgate.net
1 pI of Glycine with Titration curve Download Scientific Diagram Titration Curve Pi The isoelectric points range from 5.5 to 6.2. If we only add half as much base, only. Titration curves show the neutralization of these acids by added base, and the change in ph. How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of the. Titration Curve Pi.
From www.coursehero.com
[Solved] .The titration curve in Figure A refers to an amino acid that Titration Curve Pi If we only add half as much base, only. The isoelectric points range from 5.5 to 6.2. These curves allow the prediction of protonation states, charges, and isoelectric points. Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. Titration curves show the neutralization of these acids by added base, and. Titration Curve Pi.
From www.chegg.com
Solved Draw the titration curve for the peptide above on the Titration Curve Pi From the curve we can infer that the $\mathrm{pi}$ is simply the average of the two $\mathrm pk_\mathrm a$ values of the two carboxylic acid groups. The isoelectric points range from 5.5 to 6.2. How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of. Titration Curve Pi.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curve Pi How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of the carboxyl (2.34) and ammonium (9.60) groups. The isoelectric point refers to the ph at which. Titration curves show the neutralization of these acids by added base, and the change in ph. These curves. Titration Curve Pi.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Titration Curve Pi Titration curves show the neutralization of these acids by added base, and the change in ph. Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. The isoelectric points range from 5.5 to 6.2. If we only add half as much base, only. How to determine pi from the curve •. Titration Curve Pi.
From www.youtube.com
Ch.3 Amino Acid part 5 Titration curve of basic amino acids (Histidine Titration Curve Pi The isoelectric point refers to the ph at which. How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of the carboxyl (2.34) and ammonium (9.60) groups. Titration curves show the neutralization of these acids by added base, and the change in ph. Undergraduate biochemistry. Titration Curve Pi.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID1205383 Titration Curve Pi The isoelectric point refers to the ph at which. If we only add half as much base, only. Titration curves show the neutralization of these acids by added base, and the change in ph. Titration curves show the neutralization of these acids by added base, and the change in ph during the titration. The isoelectric points range from 5.5 to. Titration Curve Pi.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Pi How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of the carboxyl (2.34) and ammonium (9.60) groups. These curves allow the prediction of protonation states, charges, and isoelectric points. Undergraduate biochemistry students should have great familiarity with titration curves. Titration curves show the neutralization. Titration Curve Pi.
From chemistryguru.com.sg
Titration Curve of Amino Acid Titration Curve Pi The isoelectric point refers to the ph at which. How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of the carboxyl (2.34) and ammonium (9.60) groups. The isoelectric points range from 5.5 to 6.2. Titration curves show the neutralization of these acids by added. Titration Curve Pi.
From www.numerade.com
The following is a titration curve of histidine 10 Histidine pKa 9.17 Titration Curve Pi An essential concept in studying the titration curves of amino acids is the isoelectric point (pi). The isoelectric points range from 5.5 to 6.2. Undergraduate biochemistry students should have great familiarity with titration curves. How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of. Titration Curve Pi.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Pi How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of the carboxyl (2.34) and ammonium (9.60) groups. Undergraduate biochemistry students should have great familiarity with titration curves. The isoelectric point refers to the ph at which. Titration curves show the neutralization of these acids. Titration Curve Pi.
From ar.inspiredpencil.com
Titration Curve Acetic Acid Titration Curve Pi If we only add half as much base, only. An essential concept in studying the titration curves of amino acids is the isoelectric point (pi). How to determine pi from the curve • in diprotic amino acids such as glycine, • the pi is an average of the pk a's of the carboxyl (2.34) and ammonium (9.60) groups. Titration curves. Titration Curve Pi.
From www.slideshare.net
Chapter 3b Titration Curve Pi From the curve we can infer that the $\mathrm{pi}$ is simply the average of the two $\mathrm pk_\mathrm a$ values of the two carboxylic acid groups. An essential concept in studying the titration curves of amino acids is the isoelectric point (pi). Undergraduate biochemistry students should have great familiarity with titration curves. How to determine pi from the curve •. Titration Curve Pi.