What Is The Difference Between Binding Energy And Ionization Energy . Binding energy and ionization energy are two fundamental concepts in physics and chemistry that play crucial roles in. Ionization energies are equal to or. 1st ionization energy is the energy that enables the reaction x x + + e −. 2nd ionization energy is the energy that enables the reaction x + x 2+ +. Binding energy is the amount of energy required to dismantle the system's particles into individual particle. The question is, how might a measured binding energy differ from an ionization energy? Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy of hydrogen? Binding energy is usually used in the context of nuclear physics (fission/fusion calculations) and as a measure of the stability of nuclei. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. Ionization energy is the minimum.
from pediaa.com
Binding energy is usually used in the context of nuclear physics (fission/fusion calculations) and as a measure of the stability of nuclei. 1st ionization energy is the energy that enables the reaction x x + + e −. Ionization energies are equal to or. 2nd ionization energy is the energy that enables the reaction x + x 2+ +. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. Binding energy is the amount of energy required to dismantle the system's particles into individual particle. Ionization energy is the minimum. Binding energy and ionization energy are two fundamental concepts in physics and chemistry that play crucial roles in. The question is, how might a measured binding energy differ from an ionization energy? Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy of hydrogen?
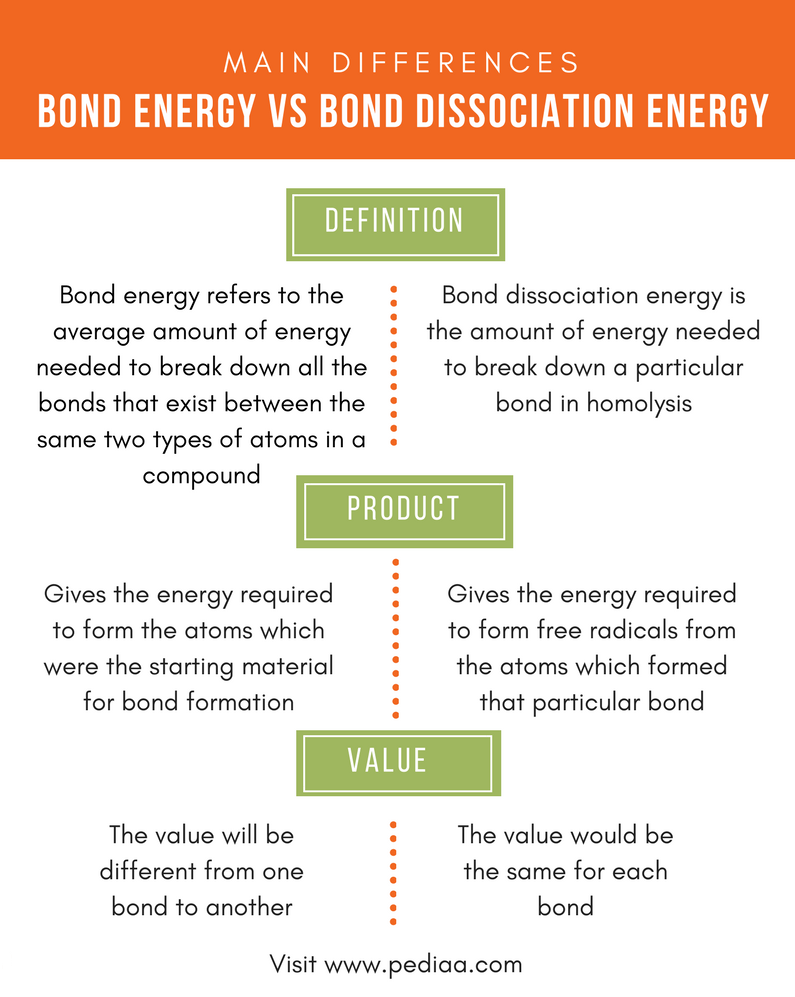
Difference Between Bond Energy and Bond Dissociation Energy
What Is The Difference Between Binding Energy And Ionization Energy Ionization energies are equal to or. Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy of hydrogen? Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. 1st ionization energy is the energy that enables the reaction x x + + e −. Binding energy is the amount of energy required to dismantle the system's particles into individual particle. Ionization energy is the minimum. Ionization energies are equal to or. 2nd ionization energy is the energy that enables the reaction x + x 2+ +. Binding energy and ionization energy are two fundamental concepts in physics and chemistry that play crucial roles in. Binding energy is usually used in the context of nuclear physics (fission/fusion calculations) and as a measure of the stability of nuclei. The question is, how might a measured binding energy differ from an ionization energy?
From pediaa.com
Difference Between Electron Gain Enthalpy and Electronegativity What Is The Difference Between Binding Energy And Ionization Energy 2nd ionization energy is the energy that enables the reaction x + x 2+ +. Binding energy and ionization energy are two fundamental concepts in physics and chemistry that play crucial roles in. The question is, how might a measured binding energy differ from an ionization energy? Binding energy is the amount of energy required to dismantle the system's particles. What Is The Difference Between Binding Energy And Ionization Energy.
From sukachem.blogspot.com
Suka Chemistry Electronegativity and Ionization Energy What Is The Difference Between Binding Energy And Ionization Energy 1st ionization energy is the energy that enables the reaction x x + + e −. Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy of hydrogen? Binding energy is the amount of energy required to dismantle the system's particles into individual particle. Binding energy and ionization energy are two fundamental. What Is The Difference Between Binding Energy And Ionization Energy.
From hazeldesnhsampson.blogspot.com
Explain the Ionization Energy Difference Between Sodium and Potassium What Is The Difference Between Binding Energy And Ionization Energy 1st ionization energy is the energy that enables the reaction x x + + e −. The question is, how might a measured binding energy differ from an ionization energy? Ionization energy is the minimum. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. Binding energy. What Is The Difference Between Binding Energy And Ionization Energy.
From pediaa.com
Difference Between Electron Affinity and Ionization Energy Definition What Is The Difference Between Binding Energy And Ionization Energy 2nd ionization energy is the energy that enables the reaction x + x 2+ +. Binding energy is the amount of energy required to dismantle the system's particles into individual particle. Ionization energies are equal to or. Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy of hydrogen? Ionization energy is. What Is The Difference Between Binding Energy And Ionization Energy.
From www.pinterest.com
What Is Ionization Energy? Definition and Trend Ionization energy What Is The Difference Between Binding Energy And Ionization Energy 2nd ionization energy is the energy that enables the reaction x + x 2+ +. Ionization energies are equal to or. Binding energy is usually used in the context of nuclear physics (fission/fusion calculations) and as a measure of the stability of nuclei. Binding energy is the amount of energy required to dismantle the system's particles into individual particle. Binding. What Is The Difference Between Binding Energy And Ionization Energy.
From www.universetoday.com
What is Binding Energy? Universe Today What Is The Difference Between Binding Energy And Ionization Energy 2nd ionization energy is the energy that enables the reaction x + x 2+ +. Binding energy is usually used in the context of nuclear physics (fission/fusion calculations) and as a measure of the stability of nuclei. Binding energy is the amount of energy required to dismantle the system's particles into individual particle. 1st ionization energy is the energy that. What Is The Difference Between Binding Energy And Ionization Energy.
From pressbooks.online.ucf.edu
Nuclear Binding Energy University Physics Volume 3 What Is The Difference Between Binding Energy And Ionization Energy Ionization energies are equal to or. The question is, how might a measured binding energy differ from an ionization energy? 2nd ionization energy is the energy that enables the reaction x + x 2+ +. Binding energy is the amount of energy required to dismantle the system's particles into individual particle. Binding energy is usually used in the context of. What Is The Difference Between Binding Energy And Ionization Energy.
From www.youtube.com
Difference between Electron affinity and Ionization Energy What Is The Difference Between Binding Energy And Ionization Energy 2nd ionization energy is the energy that enables the reaction x + x 2+ +. Ionization energy is the minimum. 1st ionization energy is the energy that enables the reaction x x + + e −. Ionization energies are equal to or. Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy. What Is The Difference Between Binding Energy And Ionization Energy.
From www.britannica.com
Nuclear binding energy Definition, Formula, Mass Defect, & Graph What Is The Difference Between Binding Energy And Ionization Energy Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy of hydrogen? Binding energy is usually used in the context of nuclear physics (fission/fusion calculations) and as a measure of the stability of nuclei. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom,. What Is The Difference Between Binding Energy And Ionization Energy.
From www.3ditalian.com
区别Between Ionization Energy and Binding Energy Compare the Difference What Is The Difference Between Binding Energy And Ionization Energy Binding energy is usually used in the context of nuclear physics (fission/fusion calculations) and as a measure of the stability of nuclei. Ionization energies are equal to or. Binding energy and ionization energy are two fundamental concepts in physics and chemistry that play crucial roles in. Why is the binding energy of the electron in atomic hydrogen not the same. What Is The Difference Between Binding Energy And Ionization Energy.
From www.differencebetween.com
Difference Between First and Second Ionization Energy (I1E vs I2E What Is The Difference Between Binding Energy And Ionization Energy The question is, how might a measured binding energy differ from an ionization energy? Ionization energies are equal to or. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. Why is the binding energy of the electron in atomic hydrogen not the same as the ionization. What Is The Difference Between Binding Energy And Ionization Energy.
From www.slideserve.com
PPT Binding energy in atoms and nuclei PowerPoint Presentation, free What Is The Difference Between Binding Energy And Ionization Energy 2nd ionization energy is the energy that enables the reaction x + x 2+ +. Ionization energy is the minimum. Ionization energies are equal to or. Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy of hydrogen? Binding energy is usually used in the context of nuclear physics (fission/fusion calculations) and. What Is The Difference Between Binding Energy And Ionization Energy.
From loemgaamq.blob.core.windows.net
Bromine Higher Ionization Energy at Kevin Hurt blog What Is The Difference Between Binding Energy And Ionization Energy Binding energy is the amount of energy required to dismantle the system's particles into individual particle. Ionization energies are equal to or. Binding energy is usually used in the context of nuclear physics (fission/fusion calculations) and as a measure of the stability of nuclei. Why is the binding energy of the electron in atomic hydrogen not the same as the. What Is The Difference Between Binding Energy And Ionization Energy.
From www.slideserve.com
PPT Ionization Energy PowerPoint Presentation, free download ID5119239 What Is The Difference Between Binding Energy And Ionization Energy Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy of hydrogen? Binding energy is the amount of energy required to dismantle the system's particles into individual particle. 2nd. What Is The Difference Between Binding Energy And Ionization Energy.
From www.animalia-life.club
Second Ionization Energy Periodic Table What Is The Difference Between Binding Energy And Ionization Energy Binding energy and ionization energy are two fundamental concepts in physics and chemistry that play crucial roles in. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy of. What Is The Difference Between Binding Energy And Ionization Energy.
From pediaa.com
Difference Between Electronegativity and Electron Affinity Definition What Is The Difference Between Binding Energy And Ionization Energy Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. 1st ionization energy is the energy that enables the reaction x x + + e −. Binding energy is the amount of energy required to dismantle the system's particles into individual particle. Binding energy and ionization energy. What Is The Difference Between Binding Energy And Ionization Energy.
From infinitylearn.com
Ionization Enthalpy Factors, Formula & Measurement Principle What Is The Difference Between Binding Energy And Ionization Energy The question is, how might a measured binding energy differ from an ionization energy? Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. 1st ionization energy is the energy that enables the reaction x x + + e −. Why is the binding energy of the. What Is The Difference Between Binding Energy And Ionization Energy.
From chem.libretexts.org
5.6 Periodic Properties of the Elements Chemistry LibreTexts What Is The Difference Between Binding Energy And Ionization Energy Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy of hydrogen? 2nd ionization energy is the energy that enables the reaction x + x 2+ +. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. Binding energy. What Is The Difference Between Binding Energy And Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps What Is The Difference Between Binding Energy And Ionization Energy Ionization energy is the minimum. 1st ionization energy is the energy that enables the reaction x x + + e −. Binding energy and ionization energy are two fundamental concepts in physics and chemistry that play crucial roles in. Binding energy is the amount of energy required to dismantle the system's particles into individual particle. Why is the binding energy. What Is The Difference Between Binding Energy And Ionization Energy.
From www.globalsino.com
Comparison between Valence (Band) and Core Electrons What Is The Difference Between Binding Energy And Ionization Energy Binding energy is the amount of energy required to dismantle the system's particles into individual particle. 1st ionization energy is the energy that enables the reaction x x + + e −. Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy of hydrogen? Ionization energy is the minimum. Binding energy is. What Is The Difference Between Binding Energy And Ionization Energy.
From byjus.com
Calculate the ionization energy and separation energy for Li2+ ion if What Is The Difference Between Binding Energy And Ionization Energy 1st ionization energy is the energy that enables the reaction x x + + e −. Binding energy is the amount of energy required to dismantle the system's particles into individual particle. Ionization energies are equal to or. Binding energy is usually used in the context of nuclear physics (fission/fusion calculations) and as a measure of the stability of nuclei.. What Is The Difference Between Binding Energy And Ionization Energy.
From www.youtube.com
Binding energy YouTube What Is The Difference Between Binding Energy And Ionization Energy Binding energy is the amount of energy required to dismantle the system's particles into individual particle. 2nd ionization energy is the energy that enables the reaction x + x 2+ +. Binding energy is usually used in the context of nuclear physics (fission/fusion calculations) and as a measure of the stability of nuclei. The question is, how might a measured. What Is The Difference Between Binding Energy And Ionization Energy.
From www.differencebetween.com
Difference Between Electronegativity and Ionization Energy Compare What Is The Difference Between Binding Energy And Ionization Energy Binding energy is usually used in the context of nuclear physics (fission/fusion calculations) and as a measure of the stability of nuclei. 2nd ionization energy is the energy that enables the reaction x + x 2+ +. Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion.. What Is The Difference Between Binding Energy And Ionization Energy.
From pediaa.com
Difference Between Bond Energy and Bond Dissociation Energy What Is The Difference Between Binding Energy And Ionization Energy Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. 1st ionization energy is the energy that enables the reaction x x + + e −. Binding energy is usually used in the context of nuclear physics (fission/fusion calculations) and as a measure of the stability of. What Is The Difference Between Binding Energy And Ionization Energy.
From www.youtube.com
Mass Defect and Binding Energy IB Physics YouTube What Is The Difference Between Binding Energy And Ionization Energy Binding energy is the amount of energy required to dismantle the system's particles into individual particle. Ionization energy is the minimum. Binding energy and ionization energy are two fundamental concepts in physics and chemistry that play crucial roles in. 1st ionization energy is the energy that enables the reaction x x + + e −. Binding energy is usually used. What Is The Difference Between Binding Energy And Ionization Energy.
From www.youtube.com
CHEM 101 Photoelectric Effect Threshold Frequency and Binding Energy What Is The Difference Between Binding Energy And Ionization Energy 2nd ionization energy is the energy that enables the reaction x + x 2+ +. Binding energy is usually used in the context of nuclear physics (fission/fusion calculations) and as a measure of the stability of nuclei. Binding energy is the amount of energy required to dismantle the system's particles into individual particle. The question is, how might a measured. What Is The Difference Between Binding Energy And Ionization Energy.
From sciencenotes.org
What Is Ionization Energy? Definition and Trend What Is The Difference Between Binding Energy And Ionization Energy 1st ionization energy is the energy that enables the reaction x x + + e −. Ionization energies are equal to or. Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy of hydrogen? Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom,. What Is The Difference Between Binding Energy And Ionization Energy.
From chemistry.stackexchange.com
quantum chemistry Binding energy vs. ionization energy Chemistry What Is The Difference Between Binding Energy And Ionization Energy Ionization energy is the minimum. Ionization energies are equal to or. Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy of hydrogen? Binding energy is the amount of energy required to dismantle the system's particles into individual particle. Binding energy and ionization energy are two fundamental concepts in physics and chemistry. What Is The Difference Between Binding Energy And Ionization Energy.
From www.slideserve.com
PPT Ionization Energy PowerPoint Presentation, free download ID6548427 What Is The Difference Between Binding Energy And Ionization Energy Electron binding energy, also called ionization potential, is the energy required to remove an electron from an atom, a molecule, or an ion. 2nd ionization energy is the energy that enables the reaction x + x 2+ +. Binding energy and ionization energy are two fundamental concepts in physics and chemistry that play crucial roles in. Binding energy is usually. What Is The Difference Between Binding Energy And Ionization Energy.
From ar.inspiredpencil.com
Ionization Energy List What Is The Difference Between Binding Energy And Ionization Energy Binding energy and ionization energy are two fundamental concepts in physics and chemistry that play crucial roles in. 1st ionization energy is the energy that enables the reaction x x + + e −. Ionization energies are equal to or. The question is, how might a measured binding energy differ from an ionization energy? Electron binding energy, also called ionization. What Is The Difference Between Binding Energy And Ionization Energy.
From www.slideserve.com
PPT 13.1 Properties of nucleus 13.2 Binding energy and mass defect What Is The Difference Between Binding Energy And Ionization Energy Binding energy is the amount of energy required to dismantle the system's particles into individual particle. The question is, how might a measured binding energy differ from an ionization energy? Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy of hydrogen? Binding energy and ionization energy are two fundamental concepts in. What Is The Difference Between Binding Energy And Ionization Energy.
From chemistry.stackexchange.com
physical chemistry Work function and ionization energy Chemistry What Is The Difference Between Binding Energy And Ionization Energy Binding energy and ionization energy are two fundamental concepts in physics and chemistry that play crucial roles in. Binding energy is the amount of energy required to dismantle the system's particles into individual particle. Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy of hydrogen? Ionization energies are equal to or.. What Is The Difference Between Binding Energy And Ionization Energy.
From pediaa.com
Difference Between Atomic Energy and Nuclear Energy Definition, Types What Is The Difference Between Binding Energy And Ionization Energy Binding energy is usually used in the context of nuclear physics (fission/fusion calculations) and as a measure of the stability of nuclei. Binding energy and ionization energy are two fundamental concepts in physics and chemistry that play crucial roles in. Ionization energies are equal to or. Binding energy is the amount of energy required to dismantle the system's particles into. What Is The Difference Between Binding Energy And Ionization Energy.
From www.numerade.com
SOLVED 11. What is the difference between binding energy and What Is The Difference Between Binding Energy And Ionization Energy Why is the binding energy of the electron in atomic hydrogen not the same as the ionization energy of hydrogen? 2nd ionization energy is the energy that enables the reaction x + x 2+ +. Binding energy is the amount of energy required to dismantle the system's particles into individual particle. Ionization energy is the minimum. Binding energy and ionization. What Is The Difference Between Binding Energy And Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps What Is The Difference Between Binding Energy And Ionization Energy 1st ionization energy is the energy that enables the reaction x x + + e −. The question is, how might a measured binding energy differ from an ionization energy? Binding energy is usually used in the context of nuclear physics (fission/fusion calculations) and as a measure of the stability of nuclei. Binding energy is the amount of energy required. What Is The Difference Between Binding Energy And Ionization Energy.