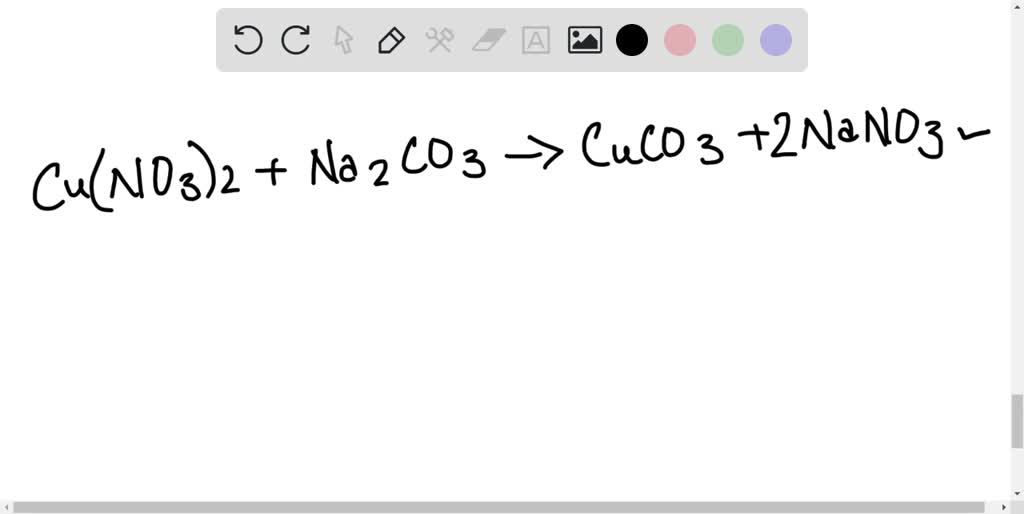Copper Ii Nitrate Balanced Equation . One mole of solid copper plus two moles of aqueous silver nitrate produce. limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. 2 cu (no3)2 → 2 cuo + 4 no2 + o2. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. 3 no2 + h2o → 2 hno3 + no. Treatment of copper (ii) nitrate. — in this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. — this chemical equation means: the equations are as follows: Three moles of copper [cu] and.
from www.numerade.com
the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. One mole of solid copper plus two moles of aqueous silver nitrate produce. — in this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. Three moles of copper [cu] and. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. 2 cu (no3)2 → 2 cuo + 4 no2 + o2. — this chemical equation means: limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. the equations are as follows:
SOLVEDWrite an overall balanced equation for the precipitation
Copper Ii Nitrate Balanced Equation Three moles of copper [cu] and. — in this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Treatment of copper (ii) nitrate. 2 cu (no3)2 → 2 cuo + 4 no2 + o2. Three moles of copper [cu] and. the equations are as follows: One mole of solid copper plus two moles of aqueous silver nitrate produce. — this chemical equation means: the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. 3 no2 + h2o → 2 hno3 + no. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole.
From www.numerade.com
SOLVED 1. When copper (II) chloride reacts with sodium nitrate, copper Copper Ii Nitrate Balanced Equation limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. — this chemical equation means: — in this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. 2 cu (no3)2 → 2 cuo + 4 no2 + o2. the equations are as follows:. Copper Ii Nitrate Balanced Equation.
From brainly.in
Balance the equation Copper Hydroxide + nitric acid …… Copper nitrate Copper Ii Nitrate Balanced Equation limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. 3 no2 + h2o → 2 hno3 + no. the equation above indicates that one mole of solid copper is reacting with two moles of. Copper Ii Nitrate Balanced Equation.
From www.toppr.com
On heating blue coloured powder of copper (II) nitrate in a boiling Copper Ii Nitrate Balanced Equation the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. Three moles of copper [cu] and. One mole of solid copper plus two moles of aqueous silver nitrate produce. the equations are as follows: Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical). Copper Ii Nitrate Balanced Equation.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Copper Ii Nitrate Balanced Equation One mole of solid copper plus two moles of aqueous silver nitrate produce. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. limiting reagent can be computed for a balanced equation. Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED 1. Write the balanced chemical equation for the reaction of Copper Ii Nitrate Balanced Equation — this chemical equation means: — in this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. One mole of solid copper plus two moles of aqueous silver nitrate produce. limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Three moles of copper. Copper Ii Nitrate Balanced Equation.
From www.youtube.com
How to Write the Formula for Copper (II) nitrate YouTube Copper Ii Nitrate Balanced Equation Three moles of copper [cu] and. One mole of solid copper plus two moles of aqueous silver nitrate produce. Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. the equations are as follows: the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce. Copper Ii Nitrate Balanced Equation.
From www.youtube.com
What kind of reaction is Copper(II) nitrate (Cu(NO3)2) and Sodium Copper Ii Nitrate Balanced Equation One mole of solid copper plus two moles of aqueous silver nitrate produce. 3 no2 + h2o → 2 hno3 + no. Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. . Copper Ii Nitrate Balanced Equation.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Copper Ii Nitrate Balanced Equation — in this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. 2 cu (no3)2 → 2 cuo + 4 no2 + o2. Three moles of copper [cu] and. the equation. Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVEDWhen the following molecular equation is balanced using the sina Copper Ii Nitrate Balanced Equation 2 cu (no3)2 → 2 cuo + 4 no2 + o2. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. One mole of solid copper plus two moles of aqueous silver nitrate. Copper Ii Nitrate Balanced Equation.
From exopnayqj.blob.core.windows.net
Silver Nitrate Copper Wire Balanced Equation at Maria Samuels blog Copper Ii Nitrate Balanced Equation 2 cu (no3)2 → 2 cuo + 4 no2 + o2. — this chemical equation means: — in this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. the equations are as follows: Treatment of copper (ii) nitrate. the equation above indicates that one mole of solid copper is reacting with two moles. Copper Ii Nitrate Balanced Equation.
From www.youtube.com
How to Balance Cu(NO3)2 = CuO + NO2 + O2 Copper (II) nitrate Copper Ii Nitrate Balanced Equation One mole of solid copper plus two moles of aqueous silver nitrate produce. Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. the equation above indicates that one mole of solid. Copper Ii Nitrate Balanced Equation.
From www.chegg.com
Solved 6. Write the balanced equation for when copper (II) Copper Ii Nitrate Balanced Equation 3 no2 + h2o → 2 hno3 + no. — in this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. Three moles of copper [cu] and. limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. 2 cu (no3)2 → 2 cuo + 4. Copper Ii Nitrate Balanced Equation.
From www.meritnation.com
Write balanced chemical equations for the conversions A,B C A Copper Copper Ii Nitrate Balanced Equation One mole of solid copper plus two moles of aqueous silver nitrate produce. 2 cu (no3)2 → 2 cuo + 4 no2 + o2. Three moles of copper [cu] and. 3 no2 + h2o → 2 hno3 + no. — in this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. Copper + nitric acid =. Copper Ii Nitrate Balanced Equation.
From www.chegg.com
Solved When copper (II) chloride reacts with sodium nitrate, Copper Ii Nitrate Balanced Equation Treatment of copper (ii) nitrate. limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. One mole of solid copper plus two moles of aqueous silver nitrate produce. the equations are as follows: — this chemical equation means: 2 cu (no3)2 → 2 cuo + 4 no2. Copper Ii Nitrate Balanced Equation.
From www.numerade.com
The reaction between solid copper and aqueous silver nitrate produces Copper Ii Nitrate Balanced Equation Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. — in this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. — this chemical equation means: 3. Copper Ii Nitrate Balanced Equation.
From www.chegg.com
Solved Write the balanced equation for when copper(II) Copper Ii Nitrate Balanced Equation One mole of solid copper plus two moles of aqueous silver nitrate produce. 3 no2 + h2o → 2 hno3 + no. Three moles of copper [cu] and. — this chemical equation means: the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. —. Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED 1. When copper(II) chloride reacts with sodium nitrate, copper Copper Ii Nitrate Balanced Equation — in this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. Three moles of copper [cu] and. One mole of solid copper plus two moles of aqueous silver nitrate produce. Treatment of copper (ii) nitrate. 2 cu (no3)2 → 2 cuo + 4 no2 + o2. the equation above indicates that one mole of. Copper Ii Nitrate Balanced Equation.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Copper Ii Nitrate Balanced Equation the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. One mole of solid copper plus two moles of aqueous silver nitrate produce. Three moles of copper [cu] and. the equations are as follows: the equation above indicates that one mole of solid copper. Copper Ii Nitrate Balanced Equation.
From www.doubtnut.com
On heating blue coloured powder of copper (II) nitrate in a boiling Copper Ii Nitrate Balanced Equation the equations are as follows: One mole of solid copper plus two moles of aqueous silver nitrate produce. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. 3 no2 + h2o. Copper Ii Nitrate Balanced Equation.
From www.youtube.com
Write the balanced chemical equation of the following word equation Copper Ii Nitrate Balanced Equation — in this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. 3 no2 + h2o → 2 hno3. Copper Ii Nitrate Balanced Equation.
From www.youtube.com
Cu+HNO3=Cu(NO3)2+NO2+H2O Balanced EquationCopper+Nitric acid=Copper Copper Ii Nitrate Balanced Equation the equations are as follows: Treatment of copper (ii) nitrate. 3 no2 + h2o → 2 hno3 + no. — this chemical equation means: the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. the equation above indicates that one mole of solid. Copper Ii Nitrate Balanced Equation.
From testbook.com
Copper (II) Nitrate Formula Learn Structure, Properties, Uses Copper Ii Nitrate Balanced Equation One mole of solid copper plus two moles of aqueous silver nitrate produce. limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. 3 no2 + h2o → 2 hno3 + no. — this chemical equation means: — in this video we'll balance the equation cu(no3)2 =. Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED Copper (II) nitrate and sodium hydrogen carbonate react Copper Ii Nitrate Balanced Equation 3 no2 + h2o → 2 hno3 + no. Three moles of copper [cu] and. One mole of solid copper plus two moles of aqueous silver nitrate produce. 2 cu (no3)2 → 2 cuo + 4 no2 + o2. Treatment of copper (ii) nitrate. Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. the equation. Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED When reacting copper(II) nitrate and sodium bicarbonate, you Copper Ii Nitrate Balanced Equation the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. — in this video we'll balance the equation cu(no3)2 = cuo + no2 +. Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED Part 1 [Copper metal to Copper (II) nitrate] 2pts. Balanced Copper Ii Nitrate Balanced Equation Three moles of copper [cu] and. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. One mole of solid copper plus two moles of aqueous silver nitrate produce. the equations are as follows: limiting reagent can be computed for a balanced equation by. Copper Ii Nitrate Balanced Equation.
From shotprofessional22.gitlab.io
Beautiful Silver Nitrate And Copper Ionic Equation Edexcel Igcse Maths Copper Ii Nitrate Balanced Equation Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Treatment of copper (ii) nitrate. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to. Copper Ii Nitrate Balanced Equation.
From khushaty.blogspot.com
Copper Ii Nitrate Formula T3DB Barium nitrate What are the correct Copper Ii Nitrate Balanced Equation — in this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one. Copper Ii Nitrate Balanced Equation.
From www.coursehero.com
[Solved] What is the balanced chemical equation of Cobalt (II) nitrate Copper Ii Nitrate Balanced Equation limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. Three moles of copper [cu] and. the equations are as follows: Treatment of copper (ii) nitrate. 2 cu (no3)2 → 2 cuo + 4 no2. Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED balanced equation; Solutions of magnesium iodide and copper (II Copper Ii Nitrate Balanced Equation — this chemical equation means: Three moles of copper [cu] and. 3 no2 + h2o → 2 hno3 + no. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to produce one mole. 2 cu (no3)2 → 2 cuo + 4 no2 + o2. One mole of solid. Copper Ii Nitrate Balanced Equation.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Copper Ii Nitrate Balanced Equation 2 cu (no3)2 → 2 cuo + 4 no2 + o2. Treatment of copper (ii) nitrate. One mole of solid copper plus two moles of aqueous silver nitrate produce. the equations are as follows: Three moles of copper [cu] and. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver. Copper Ii Nitrate Balanced Equation.
From lillyfersmcknight.blogspot.com
Copper Silver Nitrate Yields Copper Ii Nitrate Silver Copper Ii Nitrate Balanced Equation 2 cu (no3)2 → 2 cuo + 4 no2 + o2. Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. — this chemical equation means: — in this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. the equations are as follows: limiting reagent can be computed for a. Copper Ii Nitrate Balanced Equation.
From mungfali.com
Solved 1. Aqueous Ammonia Solution And Copper (ii) Nitrate 306 Copper Ii Nitrate Balanced Equation One mole of solid copper plus two moles of aqueous silver nitrate produce. limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. — in this video we'll balance the equation cu(no3)2 = cuo + no2 + o2. the equations are as follows: 3 no2 + h2o. Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED Give the complete ionic equation for the reaction (if any) that Copper Ii Nitrate Balanced Equation — this chemical equation means: Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. 3 no2 + h2o → 2 hno3 + no. 2 cu (no3)2 → 2 cuo + 4 no2 + o2. Treatment of copper (ii) nitrate. Three moles of copper [cu] and. — in this video we'll balance the equation cu(no3)2. Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVEDWrite an overall balanced equation for the precipitation Copper Ii Nitrate Balanced Equation Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. Treatment of copper (ii) nitrate. limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to. Copper Ii Nitrate Balanced Equation.
From www.numerade.com
SOLVED Write the balanced equation for the reaction copper(I Copper Ii Nitrate Balanced Equation Copper + nitric acid = copper(ii) nitrate + nitric oxide (radical) + water. Treatment of copper (ii) nitrate. limiting reagent can be computed for a balanced equation by entering the number of moles or weight for all reagents. the equation above indicates that one mole of solid copper is reacting with two moles of aqueous silver nitrate to. Copper Ii Nitrate Balanced Equation.