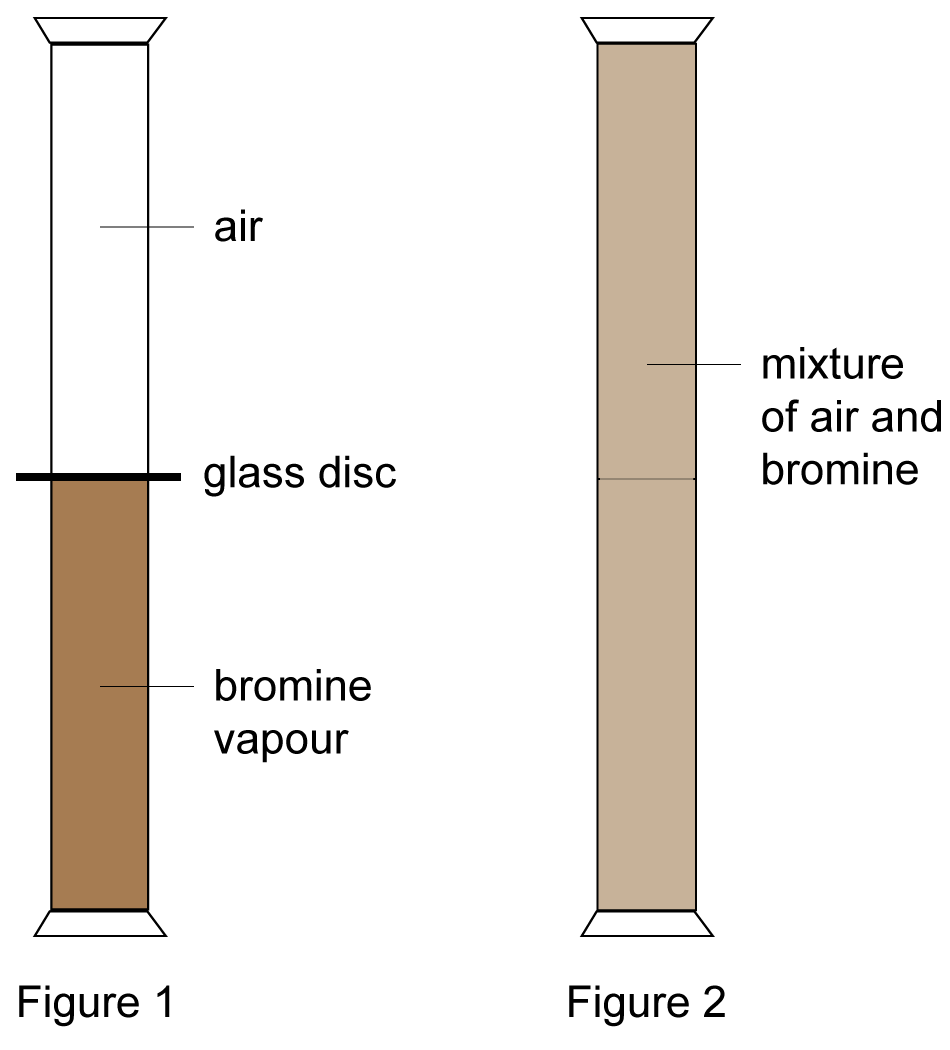Pouring Bromine Gas . The students first observe relatively sluggish diffusion of bromine into. year 9 topic 1: the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. It takes a while because bromine. if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. a classic demonstration that is explained satisfactorily by a particulate model of gases. Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching.
from gradegorilla.com
if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. a classic demonstration that is explained satisfactorily by a particulate model of gases. Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. It takes a while because bromine. The students first observe relatively sluggish diffusion of bromine into. A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. year 9 topic 1:
Gradegorilla Chemistry
Pouring Bromine Gas A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. a classic demonstration that is explained satisfactorily by a particulate model of gases. if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. year 9 topic 1: the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. It takes a while because bromine. Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. The students first observe relatively sluggish diffusion of bromine into.
From en.gazdetect.com
Bromine gas detector, Br2 GazDetect Pouring Bromine Gas the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. It takes a while because bromine. The students first observe relatively sluggish diffusion of bromine into. a classic demonstration that is explained satisfactorily by a particulate model of gases. A small amount of bromine. Pouring Bromine Gas.
From www.youtube.com
Pouring bromine YouTube Pouring Bromine Gas the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. year 9 topic 1: It takes a while because bromine. The students first. Pouring Bromine Gas.
From www.youtube.com
Bromine Gas/Liquid Equilibrium YouTube Pouring Bromine Gas A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. year 9 topic 1: It takes a while because bromine. Solids, liquids and. Pouring Bromine Gas.
From www.sciencephoto.com
Pouring Bromine Stock Image C027/9549 Science Photo Library Pouring Bromine Gas A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. the pictures. Pouring Bromine Gas.
From www.youtube.com
Bromine gas Explosion YouTube Pouring Bromine Gas A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. It takes a while because bromine. The students first observe relatively sluggish diffusion of bromine into. Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. year 9 topic 1: if you start with the bromine. Pouring Bromine Gas.
From brunofuga.adv.br
When Bromine Gas Reacts With Aqueous Sodium Hydroxide, The, 57 OFF Pouring Bromine Gas The students first observe relatively sluggish diffusion of bromine into. the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air.. Pouring Bromine Gas.
From edu.rsc.org
Handling liquid bromine and preparing bromine water Experiment RSC Pouring Bromine Gas A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. a classic demonstration that is explained satisfactorily by a particulate model of gases. It takes a while because bromine. Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. the pictures below show what happens if. Pouring Bromine Gas.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Pouring Bromine Gas year 9 topic 1: if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. The students first observe relatively sluggish diffusion of bromine into. the pictures below show what happens if a. Pouring Bromine Gas.
From exojmhggr.blob.core.windows.net
Bromine In Gas Form at Christopher Halpern blog Pouring Bromine Gas a classic demonstration that is explained satisfactorily by a particulate model of gases. It takes a while because bromine. A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing. Pouring Bromine Gas.
From www.youtube.com
shorts Pouring Bromine Gas YouTube Pouring Bromine Gas A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. a classic demonstration that is explained satisfactorily by a particulate model of gases. The students first observe relatively sluggish diffusion of bromine into. It takes a while because bromine. the pictures below show what happens if a. Pouring Bromine Gas.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Pouring Bromine Gas if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. It takes a while because bromine. year 9 topic 1: Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. A small amount of bromine liquid is allowed to evaporate into air, and. Pouring Bromine Gas.
From www.sciencephoto.com
Bromine gas Stock Image C055/5552 Science Photo Library Pouring Bromine Gas A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. It takes a while because bromine. Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. year 9 topic 1: The students first observe relatively sluggish diffusion of bromine into. if you start with the bromine. Pouring Bromine Gas.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Pouring Bromine Gas Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. a classic demonstration that is explained satisfactorily by a particulate model of gases. the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. year 9 topic 1: A small amount of bromine. Pouring Bromine Gas.
From pixels.com
Gas Diffusion Photograph by Andrew Mcclenaghan/science Photo Library Pouring Bromine Gas The students first observe relatively sluggish diffusion of bromine into. A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. year 9 topic. Pouring Bromine Gas.
From www.youtube.com
BROMINE GAS ( Br2. ) YouTube Pouring Bromine Gas the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. It takes a while because bromine. A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. year 9 topic 1: a classic. Pouring Bromine Gas.
From dxoaetgdr.blob.core.windows.net
Bromine Gas The Formula at Emma Shoulders blog Pouring Bromine Gas year 9 topic 1: the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. The students first observe relatively sluggish diffusion of bromine into. It takes a while because bromine. Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. a classic. Pouring Bromine Gas.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Pouring Bromine Gas year 9 topic 1: if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. a classic demonstration that is explained satisfactorily by a particulate model of gases. The students first observe relatively. Pouring Bromine Gas.
From www.sciencephoto.com
Bromine gas Stock Image A150/0399 Science Photo Library Pouring Bromine Gas A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. year 9 topic 1: if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. The students first observe relatively sluggish diffusion of bromine. Pouring Bromine Gas.
From ar.inspiredpencil.com
Bromine Gas Equation Pouring Bromine Gas the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. It takes a while because bromine. The students first observe. Pouring Bromine Gas.
From www.youtube.com
How to Balance P + Br2 =PBr3 (Phosphorous + Bromine gas) YouTube Pouring Bromine Gas year 9 topic 1: if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. The students first observe relatively sluggish diffusion of bromine into. A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by. Pouring Bromine Gas.
From dxoaetgdr.blob.core.windows.net
Bromine Gas The Formula at Emma Shoulders blog Pouring Bromine Gas if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. year 9 topic 1: the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. It takes a while because. Pouring Bromine Gas.
From periodictable.com
Gas in a bulb, a sample of the element Bromine in the Periodic Table Pouring Bromine Gas a classic demonstration that is explained satisfactorily by a particulate model of gases. Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. the pictures below show what happens if a test. Pouring Bromine Gas.
From fphoto.photoshelter.com
science chemistry experiment states of matter Fundamental Photographs Pouring Bromine Gas the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. The students first observe relatively sluggish diffusion of bromine into. It takes a while because bromine. a classic demonstration that is explained satisfactorily by a particulate model of gases. A small amount of bromine. Pouring Bromine Gas.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation ID Pouring Bromine Gas if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. The students first observe relatively sluggish diffusion of bromine into. a classic demonstration that is explained satisfactorily by a particulate model of gases. Solids, liquids and gasesa demonstration showing the diffusion of bromine gas.. Pouring Bromine Gas.
From fphoto.photoshelter.com
bromine phases transition chemistry element Fundamental Photographs Pouring Bromine Gas Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. It takes a while because bromine. year 9 topic 1: A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. a classic demonstration that is explained satisfactorily by a particulate model of gases. if you. Pouring Bromine Gas.
From gradegorilla.com
Gradegorilla Chemistry Pouring Bromine Gas a classic demonstration that is explained satisfactorily by a particulate model of gases. It takes a while because bromine. Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. The students first observe relatively sluggish diffusion of bromine into. A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed. Pouring Bromine Gas.
From www.youtube.com
Write the Formula for Bromine gas YouTube Pouring Bromine Gas The students first observe relatively sluggish diffusion of bromine into. if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. year 9 topic 1: Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. A small amount of bromine liquid is allowed to. Pouring Bromine Gas.
From fineartamerica.com
Bromine Gas Diffusion Photograph by Martyn F. Chillmaid/science Photo Pouring Bromine Gas The students first observe relatively sluggish diffusion of bromine into. a classic demonstration that is explained satisfactorily by a particulate model of gases. the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. A small amount of bromine liquid is allowed to evaporate into. Pouring Bromine Gas.
From www.youtube.com
Best Bromine Gas Detector (Br2) YouTube Pouring Bromine Gas The students first observe relatively sluggish diffusion of bromine into. the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. if you start with the bromine vapour in the bottom gas jar, you. Pouring Bromine Gas.
From udtechnologies.com
Bromine Production Plant from sea Bittern UD Technologies Pouring Bromine Gas A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. The students first observe relatively sluggish diffusion of bromine into. if you start. Pouring Bromine Gas.
From dxoaetgdr.blob.core.windows.net
Bromine Gas The Formula at Emma Shoulders blog Pouring Bromine Gas A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. year 9 topic 1: if you start with the bromine vapour in. Pouring Bromine Gas.
From www.sciencephoto.com
Bromine gas Stock Image C023/0565 Science Photo Library Pouring Bromine Gas if you start with the bromine vapour in the bottom gas jar, you can see it slowly diffusing into the top jar containing air. The students first observe relatively sluggish diffusion of bromine into. year 9 topic 1: the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas,. Pouring Bromine Gas.
From fineartamerica.com
Bromine Gas Diffusion Photograph by Martyn F. Chillmaid/science Photo Pouring Bromine Gas year 9 topic 1: It takes a while because bromine. a classic demonstration that is explained satisfactorily by a particulate model of gases. A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed by watching. The students first observe relatively sluggish diffusion of bromine into. the pictures below. Pouring Bromine Gas.
From saroptstroy.ru
Bromine gas Security sistems Pouring Bromine Gas year 9 topic 1: the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the ammonia. Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. A small amount of bromine liquid is allowed to evaporate into air, and its rate of diffusion is followed. Pouring Bromine Gas.
From periodictable.me
Bromine Electron Configuration (Br) with Orbital Diagram Pouring Bromine Gas It takes a while because bromine. year 9 topic 1: Solids, liquids and gasesa demonstration showing the diffusion of bromine gas. a classic demonstration that is explained satisfactorily by a particulate model of gases. the pictures below show what happens if a test tube with bromine vapor is kept in the ammonia gas, given off by the. Pouring Bromine Gas.