Glp Analytical Method Validation . this review describes the procedures and documentations required for validation of glp. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. analytical method validation studies should be designed according to regulatory guidance on method validation. a full validation of a bioanalytical method 248 should be performed when establishing a bioanalytical method for the quantification of an analyte 249 in. this guideline defines key elements necessary for the validation of bioanalytical methods. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. computerised systems should be validated if they are involved in the process of generation, measurement or assessment. validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic.
from www.complianceonline.com
this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. this review describes the procedures and documentations required for validation of glp. a full validation of a bioanalytical method 248 should be performed when establishing a bioanalytical method for the quantification of an analyte 249 in. this guideline defines key elements necessary for the validation of bioanalytical methods. validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic. analytical method validation studies should be designed according to regulatory guidance on method validation. computerised systems should be validated if they are involved in the process of generation, measurement or assessment.
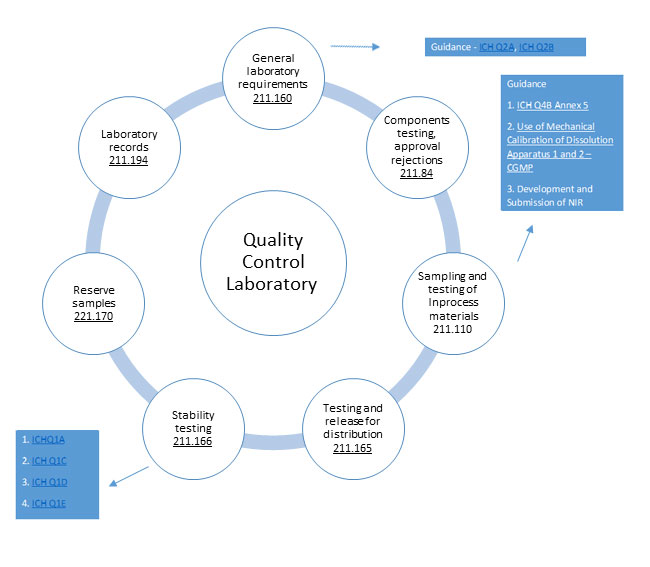
cGMP and GLP Regulations for Quality Control Labs An overview
Glp Analytical Method Validation this guideline defines key elements necessary for the validation of bioanalytical methods. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. this review describes the procedures and documentations required for validation of glp. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. computerised systems should be validated if they are involved in the process of generation, measurement or assessment. analytical method validation studies should be designed according to regulatory guidance on method validation. a full validation of a bioanalytical method 248 should be performed when establishing a bioanalytical method for the quantification of an analyte 249 in. validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic. this guideline defines key elements necessary for the validation of bioanalytical methods.
From www.bioglobax.com
ANALYTICAL TOOLKIT BioGlobaX GLP/GMP Method Development Glp Analytical Method Validation this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. this guideline defines key elements necessary for the validation of bioanalytical methods. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. a full validation of a bioanalytical method 248 should be performed when establishing. Glp Analytical Method Validation.
From www.researchgate.net
(PDF) Good Laboratory Practice (GLP) Guidelines for the Validation of Glp Analytical Method Validation the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. this guideline defines key elements necessary for the validation of bioanalytical methods. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. a full validation of a bioanalytical method 248 should be performed when establishing. Glp Analytical Method Validation.
From www.youtube.com
inar Validated Bioassays for Accelerating GLP 1 & 2 Therapeutics Glp Analytical Method Validation computerised systems should be validated if they are involved in the process of generation, measurement or assessment. this review describes the procedures and documentations required for validation of glp. this guideline defines key elements necessary for the validation of bioanalytical methods. analytical method validation studies should be designed according to regulatory guidance on method validation. . Glp Analytical Method Validation.
From gmpinsiders.com
GMP Vs GLP 10 Key Differences For Quality And Compliance Glp Analytical Method Validation analytical method validation studies should be designed according to regulatory guidance on method validation. validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic. this review describes the procedures and documentations required for validation of glp. computerised systems should be validated if they are involved in the process of generation, measurement or assessment.. Glp Analytical Method Validation.
From www.complianceonline.com
cGMP and GLP Regulations for Quality Control Labs An overview Glp Analytical Method Validation analytical method validation studies should be designed according to regulatory guidance on method validation. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic. a full validation of a bioanalytical method 248 should be performed when establishing a. Glp Analytical Method Validation.
From www.researchgate.net
Validation of the GLP1R antagonist. A) WT mice were administered a Glp Analytical Method Validation this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic. analytical method validation studies should be designed according to regulatory guidance on method validation. this guideline defines key elements necessary for the validation of bioanalytical methods. . Glp Analytical Method Validation.
From www.nanbiosis.es
GLP Analytical Validation Study in Different Animal Species Nanbiosis Glp Analytical Method Validation analytical method validation studies should be designed according to regulatory guidance on method validation. computerised systems should be validated if they are involved in the process of generation, measurement or assessment. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. this guideline defines key elements necessary for the validation. Glp Analytical Method Validation.
From www.scribd.com
GLP (Good Laboratory Practice) PDF Verification And Validation Glp Analytical Method Validation a full validation of a bioanalytical method 248 should be performed when establishing a bioanalytical method for the quantification of an analyte 249 in. this guideline defines key elements necessary for the validation of bioanalytical methods. analytical method validation studies should be designed according to regulatory guidance on method validation. this review describes the procedures and. Glp Analytical Method Validation.
From www.academia.edu
(PDF) Implementing DBS methodology for the determination of Compound A Glp Analytical Method Validation a full validation of a bioanalytical method 248 should be performed when establishing a bioanalytical method for the quantification of an analyte 249 in. validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic. computerised systems should be validated if they are involved in the process of generation, measurement or assessment. analytical method. Glp Analytical Method Validation.
From cellcarta.com
FitforPurpose GLP Validation of an IntraCellular Cytokine Staining Glp Analytical Method Validation analytical method validation studies should be designed according to regulatory guidance on method validation. this review describes the procedures and documentations required for validation of glp. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified,. Glp Analytical Method Validation.
From slideplayer.com
J. Elek , A. Simon, J. Török, A. Csontos, Cs. Ballai ppt download Glp Analytical Method Validation this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. computerised systems should be validated if they are involved in the process of generation, measurement or assessment. this review describes the procedures and documentations required. Glp Analytical Method Validation.
From studylib.net
Good Analytical Method Validation Practice Setting Up Glp Analytical Method Validation this guideline defines key elements necessary for the validation of bioanalytical methods. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic.. Glp Analytical Method Validation.
From vdocuments.mx
GLP Consulting Glp Analytical Method Validation the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. this review describes the procedures and documentations required for validation of glp. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic.. Glp Analytical Method Validation.
From pharmdguru.com
4. GLP (Good Laboratory Practice), ISO (The International Organization Glp Analytical Method Validation validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic. a full validation of a bioanalytical method 248 should be performed when establishing a bioanalytical method for the quantification of an analyte 249 in. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. this review describes the. Glp Analytical Method Validation.
From cellcarta.com
Fit for Purpose GLP Validation of an IntraCellular Staining Assay Glp Analytical Method Validation this review describes the procedures and documentations required for validation of glp. analytical method validation studies should be designed according to regulatory guidance on method validation. this guideline defines key elements necessary for the validation of bioanalytical methods. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. a. Glp Analytical Method Validation.
From www.researchgate.net
(PDF) A Global GLP Approach to Formulation Analysis Method Validation Glp Analytical Method Validation validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic. this guideline defines key elements necessary for the validation of bioanalytical methods. this review describes the procedures and documentations required for validation of glp. analytical method validation studies should be designed according to regulatory guidance on method validation. the metrology laboratory follows. Glp Analytical Method Validation.
From www.slideserve.com
PPT MPA requirements on validation of bioanalytical methods Glp Analytical Method Validation the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic. this review describes the procedures and documentations required for validation of glp. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and.. Glp Analytical Method Validation.
From www.pdffiller.com
Fillable Online GLP 14 Good Laboratory Practice for Procedure for Glp Analytical Method Validation the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. a full validation of a bioanalytical method 248 should be performed when establishing a bioanalytical method for the quantification of an analyte 249 in. analytical method validation studies should be designed according to regulatory guidance on method validation. this review describes. Glp Analytical Method Validation.
From www.certpro.co
CertPro Good Laboratory Practice Certification GLP Certification Glp Analytical Method Validation analytical method validation studies should be designed according to regulatory guidance on method validation. this review describes the procedures and documentations required for validation of glp. a full validation of a bioanalytical method 248 should be performed when establishing a bioanalytical method for the quantification of an analyte 249 in. this guideline defines key elements necessary. Glp Analytical Method Validation.
From present5.com
Validation of Analytical Methods Hua YIN Outline Glp Analytical Method Validation this guideline defines key elements necessary for the validation of bioanalytical methods. this review describes the procedures and documentations required for validation of glp. analytical method validation studies should be designed according to regulatory guidance on method validation. validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic. computerised systems should be. Glp Analytical Method Validation.
From www.researchgate.net
Typical chromatograms of GLP standard drug (A) and GLP in injection Glp Analytical Method Validation this review describes the procedures and documentations required for validation of glp. analytical method validation studies should be designed according to regulatory guidance on method validation. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical. Glp Analytical Method Validation.
From dokumen.tips
(PDF) GLP Consulting Glp Analytical Method Validation a full validation of a bioanalytical method 248 should be performed when establishing a bioanalytical method for the quantification of an analyte 249 in. this guideline defines key elements necessary for the validation of bioanalytical methods. computerised systems should be validated if they are involved in the process of generation, measurement or assessment. validated analytical methods. Glp Analytical Method Validation.
From gmpinsiders.com
Performance Characteristics In Analytical Method Validation Glp Analytical Method Validation analytical method validation studies should be designed according to regulatory guidance on method validation. a full validation of a bioanalytical method 248 should be performed when establishing a bioanalytical method for the quantification of an analyte 249 in. computerised systems should be validated if they are involved in the process of generation, measurement or assessment. the. Glp Analytical Method Validation.
From www.powershow.com
PPT Good Laboratory Practices (GLP) and USP 1058 Validation Glp Analytical Method Validation analytical method validation studies should be designed according to regulatory guidance on method validation. this review describes the procedures and documentations required for validation of glp. computerised systems should be validated if they are involved in the process of generation, measurement or assessment. validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic.. Glp Analytical Method Validation.
From www.researchgate.net
(PDF) Good Laboratory Practice (GLP) Guidelines for Change Managment Glp Analytical Method Validation validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. this guideline defines key elements necessary for the validation of bioanalytical methods. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and.. Glp Analytical Method Validation.
From www.researchgate.net
(PDF) Good Laboratory Practice (GLP) Guidelines for the Development Glp Analytical Method Validation the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. this guideline defines key elements necessary for the validation of bioanalytical methods. a full validation of a bioanalytical method 248 should be performed when establishing. Glp Analytical Method Validation.
From www.pinterest.com
This course is designed for those who perform, supervise, manage, audit Glp Analytical Method Validation computerised systems should be validated if they are involved in the process of generation, measurement or assessment. validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic. this guideline defines key elements necessary for the validation of bioanalytical methods. a full validation of a bioanalytical method 248 should be performed when establishing a. Glp Analytical Method Validation.
From www.mmsholdings.com
Good Laboratory Practice (GLP) Key Considerations & Validation Insights Glp Analytical Method Validation this guideline defines key elements necessary for the validation of bioanalytical methods. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. analytical method validation studies should be designed according to regulatory guidance on method validation. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified,. Glp Analytical Method Validation.
From www.scribd.com
GLP Quality Standards and Validation Finalized Ok PDF Verification Glp Analytical Method Validation the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. analytical method validation studies should be designed according to regulatory guidance on method validation. a full validation of a bioanalytical method 248 should be performed. Glp Analytical Method Validation.
From www.drugfuture.com
ANALYTICAL INSTRUMENT QUALIFICATION Glp Analytical Method Validation analytical method validation studies should be designed according to regulatory guidance on method validation. this review describes the procedures and documentations required for validation of glp. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. this guideline defines key elements necessary for the validation of bioanalytical methods. the. Glp Analytical Method Validation.
From microbiologyinfo.com
The Ultimate Guide to Good Laboratory Practices (GLP) Glp Analytical Method Validation this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. this guideline defines key elements necessary for the validation of bioanalytical methods. analytical method validation studies should be designed according to regulatory guidance on method validation. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified,. Glp Analytical Method Validation.
From www.youtube.com
How to GLP Validate Digital Pathology YouTube Glp Analytical Method Validation analytical method validation studies should be designed according to regulatory guidance on method validation. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. a full validation of a bioanalytical method 248 should be performed when establishing a bioanalytical method for the quantification of an analyte 249 in. this review describes. Glp Analytical Method Validation.
From www.semanticscholar.org
[PDF] A Global GLP Approach to Formulation Analysis Method Validation Glp Analytical Method Validation analytical method validation studies should be designed according to regulatory guidance on method validation. computerised systems should be validated if they are involved in the process of generation, measurement or assessment. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. this review describes the procedures and documentations required for. Glp Analytical Method Validation.
From slideplayer.com
Federaal Agentschap voor de Veiligheid van de Voedselketen 1 Validation Glp Analytical Method Validation a full validation of a bioanalytical method 248 should be performed when establishing a bioanalytical method for the quantification of an analyte 249 in. this guideline is intended to provide recommendations for the validation of bioanalytical methods for chemical and. the metrology laboratory follows this procedure to ensure that all laboratory methods selected, modified, or. this. Glp Analytical Method Validation.
From www.mmsholdings.com
Good Laboratory Practice (GLP) Key Considerations & Validation Insights Glp Analytical Method Validation analytical method validation studies should be designed according to regulatory guidance on method validation. this review describes the procedures and documentations required for validation of glp. validated analytical methods for the quantitative evaluation of analytes (i.e., drugs, including biologic. a full validation of a bioanalytical method 248 should be performed when establishing a bioanalytical method for. Glp Analytical Method Validation.