Heating Iron Sulphate . Ferrous sulfate information on this page: Learn how ferrous sulphate crystals lose water and change colour on heating, and what products are formed when they are heated further. The two solids are mixed and heated in a test tube (or ignition tube). What is formed when you leave iron(ii) sulfate in plain air? Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. When heated to about 260. Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: Upon heating to about 300 ° c, in the absence of air, a white powder is formed consisting of iron (ii) sulfate monohydrate. Solid phase heat capacity (shomate. This demonstration or class experiment shows the exothermic reaction of two elements, iron and sulfur, to form the compound, iron sulfide.
from askfilo.com
Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: Ferrous sulfate information on this page: The two solids are mixed and heated in a test tube (or ignition tube). This demonstration or class experiment shows the exothermic reaction of two elements, iron and sulfur, to form the compound, iron sulfide. What is formed when you leave iron(ii) sulfate in plain air? When heated to about 260. Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. Upon heating to about 300 ° c, in the absence of air, a white powder is formed consisting of iron (ii) sulfate monohydrate. Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. Learn how ferrous sulphate crystals lose water and change colour on heating, and what products are formed when they are heated further.
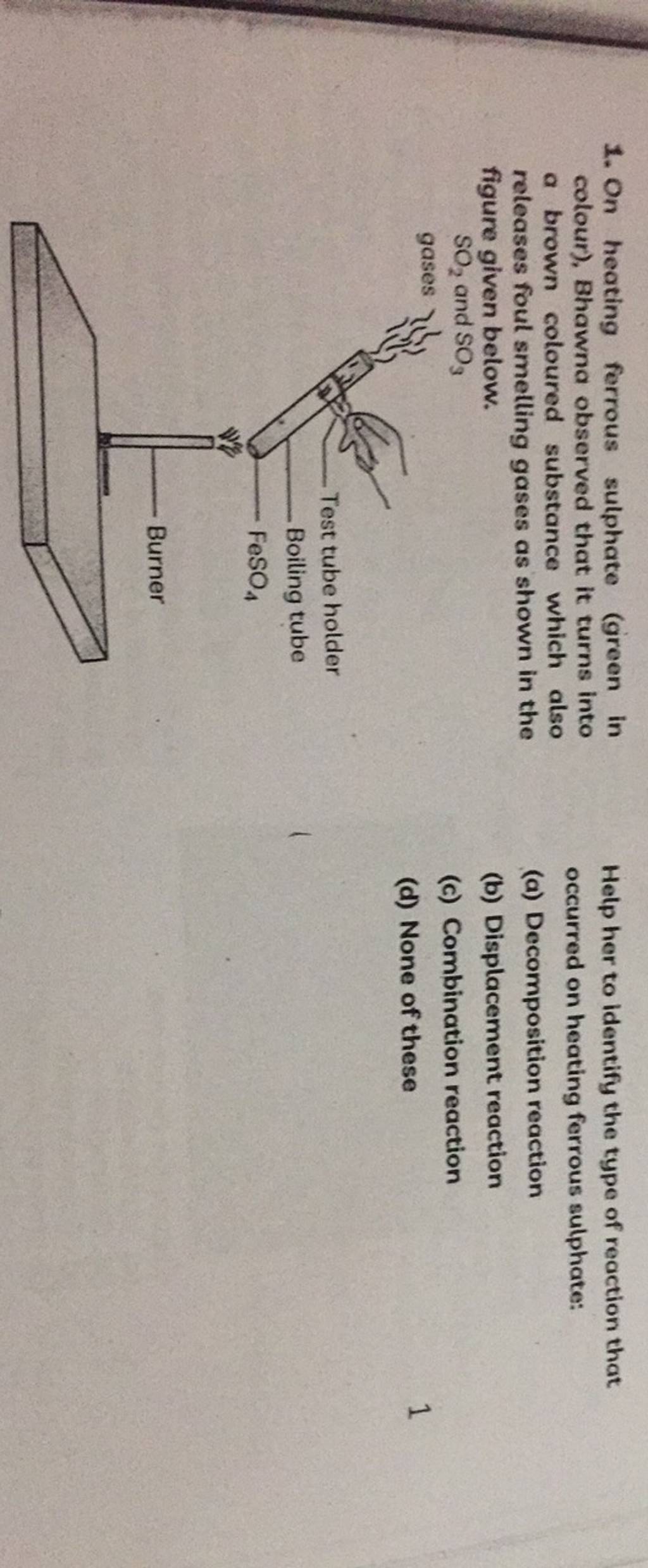
On heating ferrous sulphate Ggreen in Help her to identify the type of re..
Heating Iron Sulphate The two solids are mixed and heated in a test tube (or ignition tube). Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. The two solids are mixed and heated in a test tube (or ignition tube). Learn how ferrous sulphate crystals lose water and change colour on heating, and what products are formed when they are heated further. What is formed when you leave iron(ii) sulfate in plain air? Upon heating to about 300 ° c, in the absence of air, a white powder is formed consisting of iron (ii) sulfate monohydrate. Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. Ferrous sulfate information on this page: This demonstration or class experiment shows the exothermic reaction of two elements, iron and sulfur, to form the compound, iron sulfide. When heated to about 260. Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. Solid phase heat capacity (shomate.
From www.youtube.com
chemistry class 10 practical (to perform the action of heat on ferrous sulphate crystals Heating Iron Sulphate Learn how ferrous sulphate crystals lose water and change colour on heating, and what products are formed when they are heated further. Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: What is formed when you leave iron(ii) sulfate in plain air? The two solids are mixed and heated in a test tube (or ignition tube). Iron(ii) sulphate) or ferrous sulfate. Heating Iron Sulphate.
From www.toppr.com
12. Strong heating of ferrous sulphate leads to the formation of a brown solid and two gases Heating Iron Sulphate The two solids are mixed and heated in a test tube (or ignition tube). Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. When heated to about 260. Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. Ferrous sulfate information on this page: This demonstration or class experiment. Heating Iron Sulphate.
From www.youtube.com
heating of ferrous sulphate ferrous sulphate ko garam karna FesO4+heat Feso4 school lab work Heating Iron Sulphate This demonstration or class experiment shows the exothermic reaction of two elements, iron and sulfur, to form the compound, iron sulfide. Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: The two solids are mixed and heated in a test tube (or ignition tube). When heated to about 260. Upon heating to about 300 ° c, in the absence of air,. Heating Iron Sulphate.
From www.youtube.com
Heating Hydrated Copper Sulphate YouTube Heating Iron Sulphate Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. What is formed when you leave iron(ii) sulfate in plain air? Learn how ferrous sulphate crystals lose water and change colour on heating, and what products are formed when they are heated further. Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: Another way to prepare iron(iii) oxide is to. Heating Iron Sulphate.
From www.sciencephoto.com
Iron (II) sulphate solution Stock Image A500/0685 Science Photo Library Heating Iron Sulphate This demonstration or class experiment shows the exothermic reaction of two elements, iron and sulfur, to form the compound, iron sulfide. The two solids are mixed and heated in a test tube (or ignition tube). What is formed when you leave iron(ii) sulfate in plain air? When heated to about 260. Solid phase heat capacity (shomate. Knowing that iron(ii) is. Heating Iron Sulphate.
From brainly.in
what Happen when ferrous sulfate is heated strongly give the chemical reaction for the change Heating Iron Sulphate The two solids are mixed and heated in a test tube (or ignition tube). Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. What is formed when you leave iron(ii) sulfate in plain air? When heated to about 260. Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate,. Heating Iron Sulphate.
From www.toppr.com
2 g of ferrous sulphate crystals were heated in a hard glass test tube and observations recorded Heating Iron Sulphate Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. What is formed when you leave iron(ii) sulfate in plain air? This demonstration or class experiment shows the exothermic reaction of two elements, iron and sulfur, to form the compound, iron sulfide. Another way to prepare iron(iii) oxide. Heating Iron Sulphate.
From www.alamy.com
Iron filings sulphur heating hires stock photography and images Alamy Heating Iron Sulphate Learn how ferrous sulphate crystals lose water and change colour on heating, and what products are formed when they are heated further. The two solids are mixed and heated in a test tube (or ignition tube). This demonstration or class experiment shows the exothermic reaction of two elements, iron and sulfur, to form the compound, iron sulfide. Iron(ii) sulphate) or. Heating Iron Sulphate.
From www.youtube.com
Action of Heat on Ferrous Sulphate Crystals Reaction YouTube Heating Iron Sulphate This demonstration or class experiment shows the exothermic reaction of two elements, iron and sulfur, to form the compound, iron sulfide. Solid phase heat capacity (shomate. Ferrous sulfate information on this page: Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: When heated to about 260. Learn. Heating Iron Sulphate.
From www.toppr.com
Strong heating of ferrous sulphate leads to the formation of a brown solid and two gases. This Heating Iron Sulphate Learn how ferrous sulphate crystals lose water and change colour on heating, and what products are formed when they are heated further. The two solids are mixed and heated in a test tube (or ignition tube). Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. Upon heating to about 300 ° c, in. Heating Iron Sulphate.
From www.sciencephoto.com
Iron ii sulphate Stock Image C028/4186 Science Photo Library Heating Iron Sulphate The two solids are mixed and heated in a test tube (or ignition tube). Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. Sulfuric acid, iron(2+) salt, heptahydrate. Heating Iron Sulphate.
From www.youtube.com
(a) What is the colour of ferrous sulphate crystals? How does this colour change after heating Heating Iron Sulphate Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. Ferrous sulfate information on this page: The two solids are mixed. Heating Iron Sulphate.
From www.doubtnut.com
A few crystals of copper sulphate are heated in B/F dry boiling tu Heating Iron Sulphate Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. Ferrous sulfate information on this page: Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. Upon heating to about 300. Heating Iron Sulphate.
From www.teachoo.com
Chemical Reaction Definition, Types and Examples Class 10 Science Heating Iron Sulphate Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. This demonstration or class experiment shows the exothermic reaction of two elements, iron and sulfur, to form the compound, iron sulfide. Solid phase heat capacity (shomate. Ferrous sulfate information on this page: Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. When heated to. Heating Iron Sulphate.
From www.youtube.com
reaction action of heat on ferrous sulphate chemistry practical SSC Class 10 Heating Iron Sulphate Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. Solid phase heat capacity (shomate. Learn how ferrous sulphate crystals lose water and change colour on heating, and what products are formed when they are heated further. Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names:. Heating Iron Sulphate.
From www.toppr.com
Q. A student performed the experiment of heating ferrous sulphate cyrstals in a boiling tube. He Heating Iron Sulphate Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. What is formed when you leave iron(ii) sulfate in plain air? Learn how ferrous sulphate crystals lose water and change colour on heating, and what products are formed when they are heated further. Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: This demonstration or class experiment. Heating Iron Sulphate.
From www.youtube.com
Reaction Action of Heat on Ferrous Sulphate Crystals CBSE Practical Ajinkya Heating Iron Sulphate Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. Upon heating to about 300 ° c, in the absence of air, a white powder is formed consisting of iron (ii) sulfate monohydrate. The two solids are mixed and heated in a test tube (or ignition tube). Iron(ii) sulphate) or ferrous sulfate denotes a. Heating Iron Sulphate.
From www.youtube.com
Ferrous sulphate on heating gives (a) SO_2 and SO_3 (b) SO_2 only (c) SO_3 only (d) H_2 S only Heating Iron Sulphate Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. Upon heating to about 300 ° c, in the absence of. Heating Iron Sulphate.
From www.mdpi.com
Metals Free FullText Thermal Behavior of Hydrated Iron Sulfate in Various Atmospheres Heating Iron Sulphate Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. The two solids are mixed and heated in a test tube (or ignition tube). Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. Ferrous sulfate information on this page: Learn how ferrous sulphate crystals lose water and change colour on heating, and what products. Heating Iron Sulphate.
From www.toppr.com
Name the products formed on strongly heating ferrous sulphate crystals. What type of chemical Heating Iron Sulphate Upon heating to about 300 ° c, in the absence of air, a white powder is formed consisting of iron (ii) sulfate monohydrate. Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. When heated to about 260. Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. What is formed when you. Heating Iron Sulphate.
From www.toppr.com
On heating ferrous sulphate................and... Neem Heating Iron Sulphate Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. What is formed when you leave iron(ii) sulfate in plain air? Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: Ferrous sulfate information on this page: This demonstration or class experiment. Heating Iron Sulphate.
From www.numerade.com
SOLVED 2g of ferrous sulphate crystals are heated in a boiling tube. A. State the color of Heating Iron Sulphate Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. What is formed when you leave iron(ii) sulfate in plain air? Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: This demonstration or class experiment shows the exothermic reaction of two. Heating Iron Sulphate.
From www.toppr.com
Anhydrous ferrous sulphate on heating gives Heating Iron Sulphate Ferrous sulfate information on this page: Learn how ferrous sulphate crystals lose water and change colour on heating, and what products are formed when they are heated further. The two solids are mixed and heated in a test tube (or ignition tube). This demonstration or class experiment shows the exothermic reaction of two elements, iron and sulfur, to form the. Heating Iron Sulphate.
From askfilo.com
On heating ferrous sulphate Ggreen in Help her to identify the type of re.. Heating Iron Sulphate Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: Ferrous sulfate information on this page: This demonstration or class experiment shows the exothermic reaction of two elements, iron and sulfur, to form the compound, iron sulfide. What is formed when you leave iron(ii) sulfate in plain air? Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. When heated to. Heating Iron Sulphate.
From www.youtube.com
Science reaction chemical reaction types2 English heating ferrous Heating Iron Sulphate Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. What is formed when you leave iron(ii) sulfate in plain air? Ferrous sulfate information on this page: Learn how ferrous sulphate crystals lose water and. Heating Iron Sulphate.
From www.doubtnut.com
Ferrous sulphate on heating gives Heating Iron Sulphate Upon heating to about 300 ° c, in the absence of air, a white powder is formed consisting of iron (ii) sulfate monohydrate. Ferrous sulfate information on this page: When heated to about 260. This demonstration or class experiment shows the exothermic reaction of two elements, iron and sulfur, to form the compound, iron sulfide. What is formed when you. Heating Iron Sulphate.
From www.youtube.com
10th ,ch 1,part 5, Chemical reactions and equations,heating of ferrous sulphate,thermal Heating Iron Sulphate This demonstration or class experiment shows the exothermic reaction of two elements, iron and sulfur, to form the compound, iron sulfide. The two solids are mixed and heated in a test tube (or ignition tube). Upon heating to about 300 ° c, in the absence of air, a white powder is formed consisting of iron (ii) sulfate monohydrate. When heated. Heating Iron Sulphate.
From www.youtube.com
Copper Sulphate changes colour to white⚪ after heating🔥 YouTube Heating Iron Sulphate What is formed when you leave iron(ii) sulfate in plain air? Upon heating to about 300 ° c, in the absence of air, a white powder is formed consisting of iron (ii) sulfate monohydrate. This demonstration or class experiment shows the exothermic reaction of two elements, iron and sulfur, to form the compound, iron sulfide. The two solids are mixed. Heating Iron Sulphate.
From www.numerade.com
⏩SOLVEDThe crystals of ferrous sulphate on heating give (a)… Numerade Heating Iron Sulphate The two solids are mixed and heated in a test tube (or ignition tube). Solid phase heat capacity (shomate. Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. What is formed when you leave iron(ii) sulfate in plain air? Learn how ferrous sulphate crystals lose water and change colour on heating, and what. Heating Iron Sulphate.
From www.youtube.com
Effect of heat on ferrous sulphate crystals10thCH1SCIENCECBSENCERT AnimatedConcept in Heating Iron Sulphate Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: When heated to about 260. This demonstration or class experiment shows the exothermic reaction of two elements, iron and sulfur, to form the compound, iron sulfide. Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. The two solids are mixed and heated in a test. Heating Iron Sulphate.
From www.youtube.com
Heating Ferrous sulphate crystals Chemistry Nitika Anand chemistry scienceexperiment Heating Iron Sulphate Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. The two solids are mixed and heated in a test tube (or ignition tube). What is formed when you leave iron(ii) sulfate in plain air? Ferrous sulfate information on this page: Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. Iron(ii) sulphate). Heating Iron Sulphate.
From www.youtube.com
The action of heat on Hydrated iron II sulphate YouTube Heating Iron Sulphate Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that. Learn how ferrous sulphate crystals lose water and change colour on heating, and what products are formed when they are heated further. Upon heating to about 300 ° c, in the absence of air, a white powder is formed consisting of iron (ii) sulfate monohydrate. Solid phase heat capacity. Heating Iron Sulphate.
From rstomar3114.blogspot.com
Rusty's Biozone CHEMICAL REACTIONS AND EQUATIONS Heating Iron Sulphate The two solids are mixed and heated in a test tube (or ignition tube). When heated to about 260. Upon heating to about 300 ° c, in the absence of air, a white powder is formed consisting of iron (ii) sulfate monohydrate. This demonstration or class experiment shows the exothermic reaction of two elements, iron and sulfur, to form the. Heating Iron Sulphate.
From www.youtube.com
list any two observations when ferrous sulphate is heated in a dry test tube. sqp science q 1 Heating Iron Sulphate Upon heating to about 300 ° c, in the absence of air, a white powder is formed consisting of iron (ii) sulfate monohydrate. The two solids are mixed and heated in a test tube (or ignition tube). When heated to about 260. Ferrous sulfate information on this page: This demonstration or class experiment shows the exothermic reaction of two elements,. Heating Iron Sulphate.
From www.collegesearch.in
Ferrous Sulphate Formula Definitions, History, Physical and Chemical Properties, Structure Heating Iron Sulphate What is formed when you leave iron(ii) sulfate in plain air? Another way to prepare iron(iii) oxide is to heat iron(ii) sulfate, which decomposes to give ferric. Iron(ii) sulphate) or ferrous sulfate denotes a range of salts with the formula $\ce{feso4·xh2o}$. Sulfuric acid, iron(2+) salt, heptahydrate (1:1:7) other names: Knowing that iron(ii) is easily oxidised to iron(iii), and assuming that.. Heating Iron Sulphate.