Why Is Q Used For Heat . When we want to know the change in enthalpy. Why we use $δq$ in thermodynamics if heat $q$ is flow of energy? Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. (what's difference are we calculating here?) Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization \(\delta h_{vap}\). The use of q as a symbol for heat is derived from the french word quantité de chaleur, meaning quantity of heat. Learn how to use ideal and real. In response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present.
from slidetodoc.com
Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. The use of q as a symbol for heat is derived from the french word quantité de chaleur, meaning quantity of heat. Why we use $δq$ in thermodynamics if heat $q$ is flow of energy? Learn how to use ideal and real. In response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization \(\delta h_{vap}\). Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. (what's difference are we calculating here?) When we want to know the change in enthalpy.
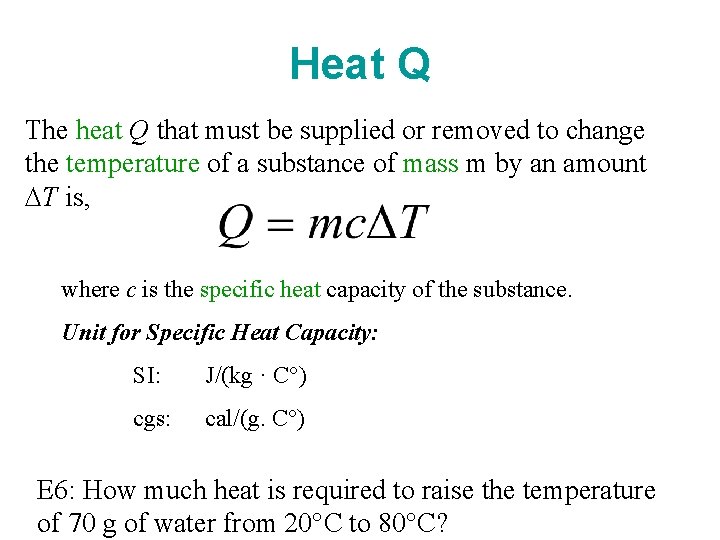
Chapter10 Temperature and Heat 1 Temperature and its
Why Is Q Used For Heat Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Why we use $δq$ in thermodynamics if heat $q$ is flow of energy? Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. When we want to know the change in enthalpy. The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization \(\delta h_{vap}\). (what's difference are we calculating here?) In response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. The use of q as a symbol for heat is derived from the french word quantité de chaleur, meaning quantity of heat. Learn how to use ideal and real.
From www.sliderbase.com
Heat Presentation Chemistry Why Is Q Used For Heat Learn how to use ideal and real. Why we use $δq$ in thermodynamics if heat $q$ is flow of energy? When we want to know the change in enthalpy. The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization \(\delta h_{vap}\). (what's difference are we. Why Is Q Used For Heat.
From www.youtube.com
Heat (w), Work (q), Change in Internal Energy (∆E) Practice Problems Why Is Q Used For Heat Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the. Why Is Q Used For Heat.
From www.youtube.com
HTPIB14B Specific Heat and Q = mcT YouTube Why Is Q Used For Heat In response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. Why we use $δq$ in thermodynamics if heat $q$ is flow of energy? Learn how to use ideal and real. Heat is an energy transfer, whereas enthalpy is a kind of energy content of. Why Is Q Used For Heat.
From courses.lumenlearning.com
Temperature Change and Heat Capacity Physics Why Is Q Used For Heat In response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. When we want to know the change in enthalpy. (what's difference are we calculating here?) Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. Why we use. Why Is Q Used For Heat.
From www.slideserve.com
PPT Heat Q vs Work W and efficiency PowerPoint Presentation, free Why Is Q Used For Heat In response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization \(\delta h_{vap}\). When we want to know the change. Why Is Q Used For Heat.
From www.thoughtco.com
Definition and Examples of Heat Energy Why Is Q Used For Heat Why we use $δq$ in thermodynamics if heat $q$ is flow of energy? Learn how to use ideal and real. In response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. The energy per unit mass required to change a substance from the liquid phase. Why Is Q Used For Heat.
From general.chemistrysteps.com
Heat Capacity and Specific Heat Why Is Q Used For Heat The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization \(\delta h_{vap}\). The use of q as a symbol for heat is derived from the french word quantité de chaleur, meaning quantity of heat. Learn how to use ideal and real. When we want to. Why Is Q Used For Heat.
From www.slideserve.com
PPT Heat (q) PowerPoint Presentation, free download ID1551407 Why Is Q Used For Heat When we want to know the change in enthalpy. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. Learn how to use ideal and real. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. The energy per unit mass required. Why Is Q Used For Heat.
From becuo.com
Heat Transfer Images & Pictures Becuo Why Is Q Used For Heat The use of q as a symbol for heat is derived from the french word quantité de chaleur, meaning quantity of heat. Why we use $δq$ in thermodynamics if heat $q$ is flow of energy? (what's difference are we calculating here?) When we want to know the change in enthalpy. Learn how to use ideal and real. The energy per. Why Is Q Used For Heat.
From www.slideserve.com
PPT Chapter 6 Thermodynamics PowerPoint Presentation, free download Why Is Q Used For Heat Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. In response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. Learn how to use ideal and real. When we want to know the change in enthalpy. Why we. Why Is Q Used For Heat.
From www.grc.nasa.gov
Heat Transfer Why Is Q Used For Heat Why we use $δq$ in thermodynamics if heat $q$ is flow of energy? When we want to know the change in enthalpy. Learn how to use ideal and real. (what's difference are we calculating here?) The use of q as a symbol for heat is derived from the french word quantité de chaleur, meaning quantity of heat. Calorimetry is an. Why Is Q Used For Heat.
From www.youtube.com
Calculating Heat (q=mcdT) YouTube Why Is Q Used For Heat Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. In response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. Learn how to use ideal and real. The energy per unit mass required to change a substance from. Why Is Q Used For Heat.
From arenahanna.wordpress.com
HEAT WORLD OF PHYSICS steps by steps to understand heat Why Is Q Used For Heat Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. In response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. Learn how to use ideal and real. The energy per unit mass. Why Is Q Used For Heat.
From www.youtube.com
General Chemistry Heat Capacity (q=smΔT) [Example 1] YouTube Why Is Q Used For Heat (what's difference are we calculating here?) Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. The use of q as a symbol for heat is derived from the french word quantité de chaleur, meaning quantity of heat. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction. Why Is Q Used For Heat.
From www.shutterstock.com
Heat Energy Formula Physics Stock Vector (Royalty Free) 2017214660 Why Is Q Used For Heat The use of q as a symbol for heat is derived from the french word quantité de chaleur, meaning quantity of heat. The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization \(\delta h_{vap}\). Why we use $δq$ in thermodynamics if heat $q$ is flow. Why Is Q Used For Heat.
From www.tessshebaylo.com
Equation For Heat Energy Absorbed Tessshebaylo Why Is Q Used For Heat Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. In response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. The use of q as a symbol for heat is derived from. Why Is Q Used For Heat.
From www.slideserve.com
PPT ENERGY PowerPoint Presentation, free download ID3232966 Why Is Q Used For Heat The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization \(\delta h_{vap}\). Why we use $δq$ in thermodynamics if heat $q$ is flow of energy? (what's difference are we calculating here?) In response to a reader query, this article traces the origins of the use. Why Is Q Used For Heat.
From www.youtube.com
Specific Heat Capacity (q=mC∆T) Examples, Practice Problems, Initial Why Is Q Used For Heat Learn how to use ideal and real. The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization \(\delta h_{vap}\). (what's difference are we calculating here?) Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. When we want to. Why Is Q Used For Heat.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID1015568 Why Is Q Used For Heat The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization \(\delta h_{vap}\). When we want to know the change in enthalpy. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. (what's difference. Why Is Q Used For Heat.
From www.slideserve.com
PPT HEAT EQUATION (in Table T) PowerPoint Presentation, free download Why Is Q Used For Heat In response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. Learn how to use ideal and real. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. Calorimetry is an application of the first law of thermodynamics to. Why Is Q Used For Heat.
From studylib.net
Heat Equation Why Is Q Used For Heat Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. When we want to know the change in enthalpy. In response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. (what's difference are. Why Is Q Used For Heat.
From www.youtube.com
Q = mcΔT and Specific Heat IB Physics YouTube Why Is Q Used For Heat (what's difference are we calculating here?) Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. The use of q as a symbol for heat is derived from the french word quantité de chaleur, meaning quantity of heat. When we want to know the change in enthalpy. Heat. Why Is Q Used For Heat.
From courses.lumenlearning.com
Introduction to the Second Law of Thermodynamics Heat Engines and Why Is Q Used For Heat (what's difference are we calculating here?) Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. In response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. Why we use $δq$ in thermodynamics. Why Is Q Used For Heat.
From www.slideshare.net
Heat energy Why Is Q Used For Heat The use of q as a symbol for heat is derived from the french word quantité de chaleur, meaning quantity of heat. The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization \(\delta h_{vap}\). Calorimetry is an application of the first law of thermodynamics to. Why Is Q Used For Heat.
From www.slideserve.com
PPT Thermochemical Calculations PowerPoint Presentation, free Why Is Q Used For Heat Learn how to use ideal and real. (what's difference are we calculating here?) The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization \(\delta h_{vap}\). In response to a reader query, this article traces the origins of the use of q and q to symbolize. Why Is Q Used For Heat.
From www.tessshebaylo.com
Equation For Heat Energy Q Tessshebaylo Why Is Q Used For Heat When we want to know the change in enthalpy. In response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. Why we use $δq$ in thermodynamics if heat $q$. Why Is Q Used For Heat.
From www.slideserve.com
PPT Unit 4 Thermochemistry & Thermodynamics PowerPoint Presentation Why Is Q Used For Heat Learn how to use ideal and real. When we want to know the change in enthalpy. The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization \(\delta h_{vap}\). Why we use $δq$ in thermodynamics if heat $q$ is flow of energy? (what's difference are we. Why Is Q Used For Heat.
From slidetodoc.com
Chapter10 Temperature and Heat 1 Temperature and its Why Is Q Used For Heat When we want to know the change in enthalpy. The use of q as a symbol for heat is derived from the french word quantité de chaleur, meaning quantity of heat. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. Why we use $δq$ in thermodynamics if heat $q$ is flow of energy?. Why Is Q Used For Heat.
From www.tessshebaylo.com
Equation For Heat Energy Q Tessshebaylo Why Is Q Used For Heat Learn how to use ideal and real. The use of q as a symbol for heat is derived from the french word quantité de chaleur, meaning quantity of heat. (what's difference are we calculating here?) The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization. Why Is Q Used For Heat.
From ispitna.blogspot.com
Q Formula Chemistry Ispitna Why Is Q Used For Heat Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. The use of q as a symbol for heat is derived from the french word quantité de chaleur, meaning quantity of heat. The energy per unit mass required to change a substance from the liquid phase to the. Why Is Q Used For Heat.
From slidetodoc.com
Heat q Heat the transfer of energy between Why Is Q Used For Heat In response to a reader query, this article traces the origins of the use of q and q to symbolize heat from 1834 to the present. Why we use $δq$ in thermodynamics if heat $q$ is flow of energy? Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. Why Is Q Used For Heat.
From www.youtube.com
Chemistry Regents Advanced Heat Calculations using Q=mCΔT YouTube Why Is Q Used For Heat The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization \(\delta h_{vap}\). (what's difference are we calculating here?) Why we use $δq$ in thermodynamics if heat $q$ is flow of energy? Heat is an energy transfer, whereas enthalpy is a kind of energy content of. Why Is Q Used For Heat.
From heattransferkarikuse.blogspot.com
Heat Transfer Heat Transfer Q Equation Why Is Q Used For Heat (what's difference are we calculating here?) Learn how to use ideal and real. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization \(\delta h_{vap}\). The use of q. Why Is Q Used For Heat.
From slidetodoc.com
Unit 3 Energy Part 2 Energy Transfer Chapter Why Is Q Used For Heat The energy per unit mass required to change a substance from the liquid phase to the vapor phase is known as the heat of vaporization \(\delta h_{vap}\). Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or. Why Is Q Used For Heat.
From www.youtube.com
Specific Heat Capacity q = mcT Everything you need to know! Chemistry Why Is Q Used For Heat The use of q as a symbol for heat is derived from the french word quantité de chaleur, meaning quantity of heat. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances.. Why Is Q Used For Heat.