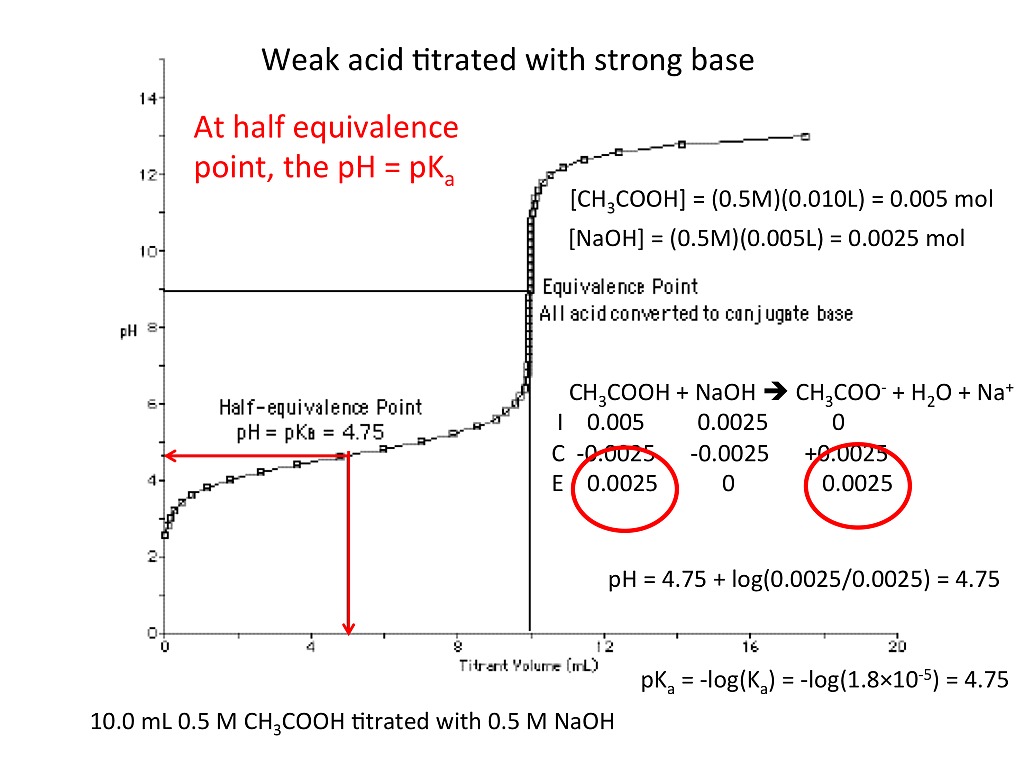
Titration Explained Simply . titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. For example, you can use. titration is a chemical process used to determine the concentration of a substance in a solution. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution.
from www.showme.com
titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. For example, you can use. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is a chemical process used to determine the concentration of a substance in a solution. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte.
Titration Curve Explained Science, Chemistry ShowMe
Titration Explained Simply titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. For example, you can use. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is a chemical process used to determine the concentration of a substance in a solution. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Explained Simply titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. a titration is a laboratory technique used to precisely measure molar concentration. Titration Explained Simply.
From www.youtube.com
Titrations Titration Calculations GCSE Chemistry Explained YouTube Titration Explained Simply It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration is the slow addition of one solution of a known concentration (called a. Titration Explained Simply.
From www.tes.com
Titrations Required Practical Sheet Teaching Resources Titration Explained Simply titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is a chemical process used to determine the concentration of a substance in a solution. titration is a way of measuring the concentration of something, usually the concentration of a substance in a. Titration Explained Simply.
From mavink.com
Titration Picture Titration Explained Simply titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is a way of measuring the concentration of something, usually the concentration of. Titration Explained Simply.
From www.pinterest.com
Titration Easy Science Learn biology, Cool science facts, Ap chemistry Titration Explained Simply titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is a way of measuring the concentration of something, usually the concentration of a substance in. Titration Explained Simply.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Explained Simply It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is a chemical process used to determine the concentration of a substance in a solution. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. For example, you can use. . Titration Explained Simply.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Titration Explained Simply It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to another solution such that it reacts under conditions. Titration Explained Simply.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Titration Explained Simply a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration involves the gradual addition of a reagent. Titration Explained Simply.
From labthink.netlify.app
What is titration and how does it work Titration Explained Simply a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration is a way of measuring the concentration. Titration Explained Simply.
From medsolut.com
Calculate titration simply explained MedSolut Titration Explained Simply For example, you can use. titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is the slow addition of one solution of. Titration Explained Simply.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Titration Explained Simply titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration is a chemical process used to determine the concentration of a substance in a solution. titration involves the gradual addition of a reagent of known concentration, known as. Titration Explained Simply.
From www.youtube.com
Conductometric Titration Principle and Introduction Easy Explanation YouTube Titration Explained Simply titration is a chemical process used to determine the concentration of a substance in a solution. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. For example, you can use. titration is the slow addition of one solution of a known concentration (called a titrant) to a. Titration Explained Simply.
From www.youtube.com
A Detailed Explanation of Titration YouTube Titration Explained Simply titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. a titration is a laboratory technique used to precisely measure molar concentration. Titration Explained Simply.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Explained Simply a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. For example, you can use. It is used in quantitative analytical chemistry to determine an. Titration Explained Simply.
From chemistry-acidsandbases.weebly.com
Titrations Titration Explained Simply a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using. Titration Explained Simply.
From exoccrkbm.blob.core.windows.net
Titration Definition Chemistry Simple at Mary McGee blog Titration Explained Simply titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. a titration is a technique where a solution of known concentration is used to determine. Titration Explained Simply.
From dokumen.tips
(PDF) Guide ABC of Easy Titration DOKUMEN.TIPS Titration Explained Simply titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is a chemical process used to determine the concentration of a substance in a solution. titration is a way of measuring the concentration of something, usually the concentration of a substance in a. Titration Explained Simply.
From www.science-revision.co.uk
Titrations Titration Explained Simply a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. titration is a chemical process used to determine the concentration of a substance in a solution. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. It is used. Titration Explained Simply.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Explained Simply It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. For. Titration Explained Simply.
From printablevozljevrc.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration Explained Simply It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. For example, you can use. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is the process in which one solution is added to another solution such. Titration Explained Simply.
From dxoradwxf.blob.core.windows.net
Titration Explained Gcse at Agnes Foster blog Titration Explained Simply titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. It is used in quantitative analytical chemistry to determine an unknown concentration. Titration Explained Simply.
From www.youtube.com
Titrations Titration Calculations A level Chemistry Explained YouTube Titration Explained Simply titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. For example, you can use. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is the slow addition of one solution of a known concentration (called a. Titration Explained Simply.
From www.youtube.com
Titration Definition, steps and formula YouTube Titration Explained Simply titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. titration is a chemical process used to determine the concentration of a substance in a solution. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. For example,. Titration Explained Simply.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Titration Explained Simply titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. For example, you can use. titration involves the gradual addition of a reagent of known. Titration Explained Simply.
From www.youtube.com
Titration Explained YouTube Titration Explained Simply titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. For example, you can use. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. titration, process of chemical analysis in which the quantity of. Titration Explained Simply.
From www.youtube.com
Titrations Explained titration chemistry stoichiometry YouTube Titration Explained Simply titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. a titration is a technique where a solution of known concentration is used to determine. Titration Explained Simply.
From www.scribd.com
Week 4. Titration Titration Chemistry Titration Explained Simply For example, you can use. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is the slow addition of one solution of a. Titration Explained Simply.
From resource.studiaacademy.com
Acids, Alkalis and Titrations Studia Academy Resources Titration Explained Simply titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. a titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. a titration is a technique where a solution of known concentration is used to. Titration Explained Simply.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Explained Simply titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. a titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. It is used in quantitative analytical chemistry to determine. Titration Explained Simply.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Explained Simply titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration, process of chemical analysis in which the quantity of some constituent of a sample is determined. titration is a chemical process. Titration Explained Simply.
From www.youtube.com
Grade 12 acids and bases titration tricks easy explained YouTube Titration Explained Simply titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration is a chemical process used to determine the concentration of a substance in a solution. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration, process. Titration Explained Simply.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID1154145 Titration Explained Simply titration is the process in which one solution is added to another solution such that it reacts under conditions in which the added volume may be accurately measured. For example, you can use. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration. Titration Explained Simply.
From www.slideserve.com
PPT Ch 16 Redox Titrations PowerPoint Presentation ID6602548 Titration Explained Simply titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. It is used in quantitative analytical chemistry to determine an unknown concentration of an identified analyte. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume. Titration Explained Simply.
From www.scribd.com
Guide ABC of Easy Titration PDF Titration Chemistry Titration Explained Simply titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration involves the gradual addition of a reagent of known concentration, known as the titrant, to a solution whose concentration needs to be. titration, process of chemical analysis in which the quantity of some. Titration Explained Simply.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Explained Simply titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. titration is the process in which one solution is added to another solution such that. Titration Explained Simply.