Does Calcium Form A Negative Ion . So na + is the sodium ion; An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. Thus, we must have two negative charges to balance the 2+ charge of the calcium ion. An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. Most monatomic anions form when a neutral. If the element has more than one possible charge, the value of the charge comes after the. It is not calcium(ii) chloride, because calcium forms only one cation when it forms an ion, and it has a characteristic charge of 2+. Positively charged ions are called cations, and negatively charged ions are called. This requires a ratio of one ca 2+ ion to two h 2 po 4. Thus, nonmetals tend to form negative ions. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Normal metals like sodium or calcium have a positive charge as $\ce{na}^+$ or $\ce{ca}^{2+}$. Transition metals have a loot of. Ca 2+ is the calcium ion.
from www.labpedia.net
Normal metals like sodium or calcium have a positive charge as $\ce{na}^+$ or $\ce{ca}^{2+}$. An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. Transition metals have a loot of. So na + is the sodium ion; Positively charged ions are called cations, and negatively charged ions are called. An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. This requires a ratio of one ca 2+ ion to two h 2 po 4. Ca 2+ is the calcium ion. Thus, nonmetals tend to form negative ions. If the element has more than one possible charge, the value of the charge comes after the.
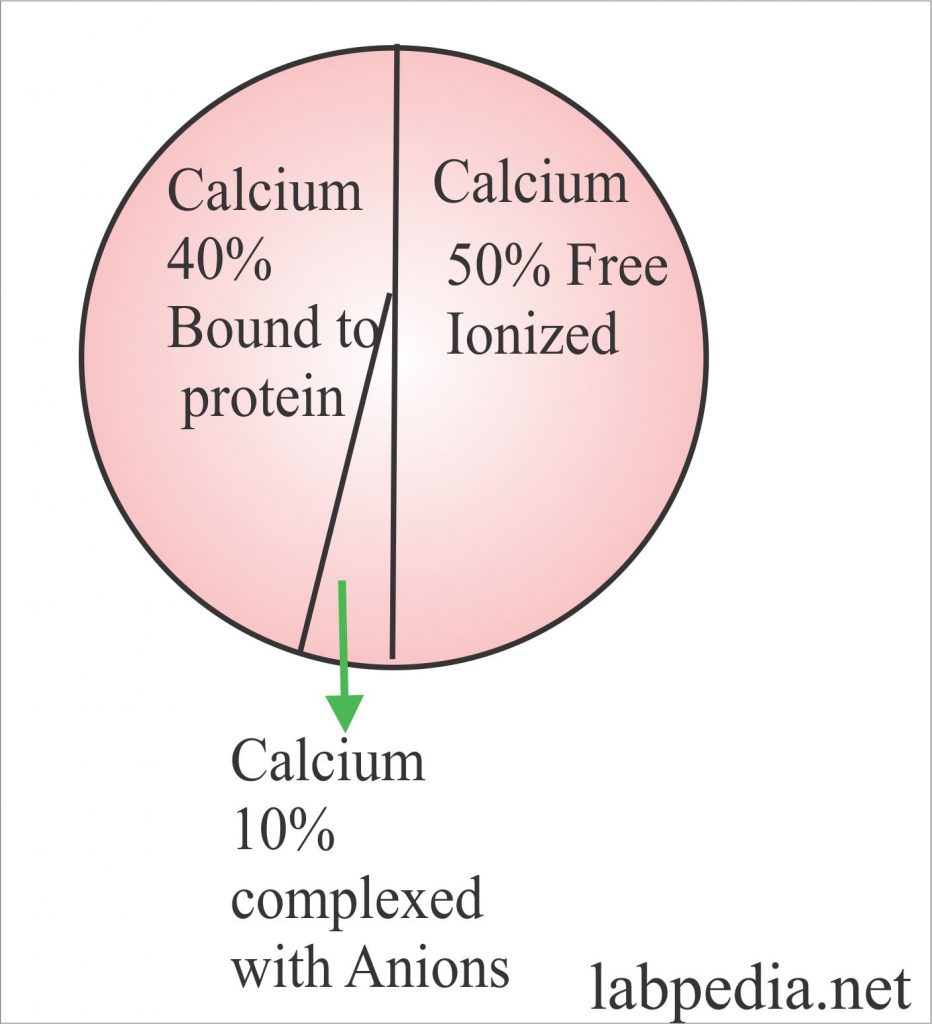
Calcium Calcium Total , Serum Calcium Part 1
Does Calcium Form A Negative Ion Normal metals like sodium or calcium have a positive charge as $\ce{na}^+$ or $\ce{ca}^{2+}$. So na + is the sodium ion; If the element has more than one possible charge, the value of the charge comes after the. Positively charged ions are called cations, and negatively charged ions are called. This requires a ratio of one ca 2+ ion to two h 2 po 4. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. Most monatomic anions form when a neutral. Transition metals have a loot of. Thus, nonmetals tend to form negative ions. Normal metals like sodium or calcium have a positive charge as $\ce{na}^+$ or $\ce{ca}^{2+}$. Ca 2+ is the calcium ion. An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. It is not calcium(ii) chloride, because calcium forms only one cation when it forms an ion, and it has a characteristic charge of 2+. Thus, we must have two negative charges to balance the 2+ charge of the calcium ion.
From www.numerade.com
SOLVED Names of Ionic Compounds Formula Positive ion Negative ion Name Does Calcium Form A Negative Ion This requires a ratio of one ca 2+ ion to two h 2 po 4. It is not calcium(ii) chloride, because calcium forms only one cation when it forms an ion, and it has a characteristic charge of 2+. An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. Ca 2+ is the. Does Calcium Form A Negative Ion.
From www.britannica.com
Calcium Definition, Properties, & Compounds Britannica Does Calcium Form A Negative Ion This requires a ratio of one ca 2+ ion to two h 2 po 4. Normal metals like sodium or calcium have a positive charge as $\ce{na}^+$ or $\ce{ca}^{2+}$. An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. Thus, nonmetals tend to form negative ions. Most monatomic anions form when a neutral.. Does Calcium Form A Negative Ion.
From www.slideserve.com
PPT I ons and B onding PowerPoint Presentation, free download ID Does Calcium Form A Negative Ion Transition metals have a loot of. If the element has more than one possible charge, the value of the charge comes after the. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Most monatomic anions form when a neutral. This requires a. Does Calcium Form A Negative Ion.
From www.youtube.com
Ca 2+ Electron Configuration (Calcium Ion) YouTube Does Calcium Form A Negative Ion Thus, we must have two negative charges to balance the 2+ charge of the calcium ion. Most monatomic anions form when a neutral. An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. This requires a ratio of one ca 2+ ion to two h 2 po 4. Normal metals like sodium or. Does Calcium Form A Negative Ion.
From animalia-life.club
Calcium Ion Lewis Dot Structure Does Calcium Form A Negative Ion Thus, we must have two negative charges to balance the 2+ charge of the calcium ion. So na + is the sodium ion; Normal metals like sodium or calcium have a positive charge as $\ce{na}^+$ or $\ce{ca}^{2+}$. This requires a ratio of one ca 2+ ion to two h 2 po 4. Transition metals have a loot of. Positively charged. Does Calcium Form A Negative Ion.
From www.slideshare.net
Chem matters ch6_ionic_bond Does Calcium Form A Negative Ion An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. This requires a ratio of one ca 2+ ion to two h 2 po 4. Most monatomic anions form when a neutral. It is not calcium(ii) chloride, because calcium forms only one cation when it forms an ion, and it has a characteristic. Does Calcium Form A Negative Ion.
From material-properties.org
Calcium Protons Neutrons Electrons Electron Configuration Does Calcium Form A Negative Ion So na + is the sodium ion; Thus, nonmetals tend to form negative ions. Transition metals have a loot of. Ca 2+ is the calcium ion. It is not calcium(ii) chloride, because calcium forms only one cation when it forms an ion, and it has a characteristic charge of 2+. An anion (negatively charged ion) forms when one or more. Does Calcium Form A Negative Ion.
From chem.libretexts.org
4.1 General Properties of Aqueous Solutions Chemistry LibreTexts Does Calcium Form A Negative Ion So na + is the sodium ion; An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. Thus, nonmetals tend to form negative ions. It is not calcium(ii) chloride, because calcium forms only one cation when it forms an ion, and it has a characteristic charge of 2+. Most monatomic anions form when. Does Calcium Form A Negative Ion.
From www.simplemed.co.uk
9. Calcium Metabolism SimpleMed Learning Medicine, Simplified Does Calcium Form A Negative Ion It is not calcium(ii) chloride, because calcium forms only one cation when it forms an ion, and it has a characteristic charge of 2+. An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. Ca 2+ is the calcium ion. This requires a ratio of one ca 2+ ion to two h 2. Does Calcium Form A Negative Ion.
From www.researchgate.net
Calcium and phosphorus homeostasis. Regulation of calcium and Does Calcium Form A Negative Ion This requires a ratio of one ca 2+ ion to two h 2 po 4. Ca 2+ is the calcium ion. An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. Thus, we must have two negative charges to balance the 2+ charge of the calcium ion. Normal metals like sodium or calcium. Does Calcium Form A Negative Ion.
From study.com
Calcium Ions Definition & Formula Video & Lesson Transcript Does Calcium Form A Negative Ion If the element has more than one possible charge, the value of the charge comes after the. Thus, we must have two negative charges to balance the 2+ charge of the calcium ion. This requires a ratio of one ca 2+ ion to two h 2 po 4. Normal metals like sodium or calcium have a positive charge as $\ce{na}^+$. Does Calcium Form A Negative Ion.
From iperiodictable.com
Calcium Electron Configuration Periodic Table Does Calcium Form A Negative Ion If the element has more than one possible charge, the value of the charge comes after the. Ca 2+ is the calcium ion. It is not calcium(ii) chloride, because calcium forms only one cation when it forms an ion, and it has a characteristic charge of 2+. Thus, we must have two negative charges to balance the 2+ charge of. Does Calcium Form A Negative Ion.
From sciencetolf.weebly.com
Periodic table with charged ions sciencetolf Does Calcium Form A Negative Ion Transition metals have a loot of. If the element has more than one possible charge, the value of the charge comes after the. It is not calcium(ii) chloride, because calcium forms only one cation when it forms an ion, and it has a characteristic charge of 2+. Normal metals like sodium or calcium have a positive charge as $\ce{na}^+$ or. Does Calcium Form A Negative Ion.
From www.studocu.com
Compound chem Compound Made Of Positive Ion Negative Ion Name and Does Calcium Form A Negative Ion An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. Normal metals like sodium or calcium have a positive charge as $\ce{na}^+$ or $\ce{ca}^{2+}$. Most monatomic anions form when a neutral. This requires a ratio of one ca 2+ ion to two h 2 po 4. Transition metals have a loot of. Thus,. Does Calcium Form A Negative Ion.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Does Calcium Form A Negative Ion Transition metals have a loot of. This requires a ratio of one ca 2+ ion to two h 2 po 4. An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. Most monatomic anions form when. Does Calcium Form A Negative Ion.
From www.sciencephoto.com
Calcium electron configuration Stock Image C029/5027 Science Does Calcium Form A Negative Ion This requires a ratio of one ca 2+ ion to two h 2 po 4. If the element has more than one possible charge, the value of the charge comes after the. An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. A cation (a positive ion) forms when a neutral atom loses. Does Calcium Form A Negative Ion.
From valenceelectrons.com
How to Write the Electron Configuration for Calcium (Ca)? Does Calcium Form A Negative Ion Positively charged ions are called cations, and negatively charged ions are called. This requires a ratio of one ca 2+ ion to two h 2 po 4. Ca 2+ is the calcium ion. Thus, we must have two negative charges to balance the 2+ charge of the calcium ion. A cation (a positive ion) forms when a neutral atom loses. Does Calcium Form A Negative Ion.
From www.youtube.com
Calcium Ion Regulation in Bone and Blood YouTube Does Calcium Form A Negative Ion Normal metals like sodium or calcium have a positive charge as $\ce{na}^+$ or $\ce{ca}^{2+}$. An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. Thus, nonmetals tend to form negative ions. If the element has more than one possible charge, the value of the charge comes after the. Thus, we must have two. Does Calcium Form A Negative Ion.
From www.pinterest.com.mx
Ion Names, Formulas and Charges Chart Teaching chemistry, Chemistry Does Calcium Form A Negative Ion Thus, nonmetals tend to form negative ions. An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. So na + is the sodium ion; Ca 2+ is the calcium ion. Normal metals like sodium or calcium have a positive charge as $\ce{na}^+$ or $\ce{ca}^{2+}$. Thus, we must have two negative charges to balance. Does Calcium Form A Negative Ion.
From www.youtube.com
How to find Protons & Electrons for the Calcium ion (Ca 2+) YouTube Does Calcium Form A Negative Ion This requires a ratio of one ca 2+ ion to two h 2 po 4. An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. Normal metals like sodium or calcium have a positive charge as $\ce{na}^+$ or $\ce{ca}^{2+}$. So na + is the sodium ion; Ca 2+ is the calcium ion. An. Does Calcium Form A Negative Ion.
From projectopenletter.com
Do Metals Form Positive Or Negative Ions Printable Form, Templates Does Calcium Form A Negative Ion Positively charged ions are called cations, and negatively charged ions are called. If the element has more than one possible charge, the value of the charge comes after the. Most monatomic anions form when a neutral. It is not calcium(ii) chloride, because calcium forms only one cation when it forms an ion, and it has a characteristic charge of 2+.. Does Calcium Form A Negative Ion.
From vdocuments.mx
Warm Up What type of ion does Calcium form? Anion or Cation Does it Does Calcium Form A Negative Ion So na + is the sodium ion; This requires a ratio of one ca 2+ ion to two h 2 po 4. Thus, we must have two negative charges to balance the 2+ charge of the calcium ion. Most monatomic anions form when a neutral. Normal metals like sodium or calcium have a positive charge as $\ce{na}^+$ or $\ce{ca}^{2+}$. A. Does Calcium Form A Negative Ion.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Does Calcium Form A Negative Ion An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. So na + is the sodium ion; An ion symbol incorporates the charge of the ion. Does Calcium Form A Negative Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Does Calcium Form A Negative Ion Ca 2+ is the calcium ion. An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. Thus, we must have two negative charges to balance the 2+ charge of the calcium ion. So na + is the sodium ion; This requires a ratio of one ca 2+ ion to two h 2 po. Does Calcium Form A Negative Ion.
From lpi.oregonstate.edu
Calcium Linus Pauling Institute Oregon State University Does Calcium Form A Negative Ion Normal metals like sodium or calcium have a positive charge as $\ce{na}^+$ or $\ce{ca}^{2+}$. An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. Thus, nonmetals tend to form negative ions. Most monatomic anions form when a neutral. This requires a ratio of one ca 2+ ion to two h 2 po 4.. Does Calcium Form A Negative Ion.
From valenceelectrons.com
How to Write the Electron Configuration for Calcium (Ca)? Does Calcium Form A Negative Ion Most monatomic anions form when a neutral. Thus, we must have two negative charges to balance the 2+ charge of the calcium ion. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. An anion (negatively charged ion) forms when one or more. Does Calcium Form A Negative Ion.
From studylambdacism.z21.web.core.windows.net
Do Metals Form Positive Or Negative Ions Does Calcium Form A Negative Ion Most monatomic anions form when a neutral. This requires a ratio of one ca 2+ ion to two h 2 po 4. Thus, nonmetals tend to form negative ions. Ca 2+ is the calcium ion. An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. Thus, we must have two negative charges to. Does Calcium Form A Negative Ion.
From www.labpedia.net
Calcium Calcium Total , Serum Calcium Part 1 Does Calcium Form A Negative Ion An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. Ca 2+ is the calcium ion. Normal metals like sodium or calcium have a positive charge as $\ce{na}^+$ or $\ce{ca}^{2+}$. It is not calcium(ii) chloride, because calcium forms only one cation when it forms an ion, and it has a characteristic charge of. Does Calcium Form A Negative Ion.
From courses.lumenlearning.com
Molecular and Ionic Compounds Chemistry for Majors Does Calcium Form A Negative Ion Ca 2+ is the calcium ion. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. Positively charged ions are called cations, and negatively charged ions. Does Calcium Form A Negative Ion.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Does Calcium Form A Negative Ion Most monatomic anions form when a neutral. An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. If the element has more than one possible charge, the value of the charge comes after the. It is not calcium(ii) chloride, because calcium forms only one cation when it forms an ion, and it has. Does Calcium Form A Negative Ion.
From www.slideserve.com
PPT Chemical Names & Formulas PowerPoint Presentation, free download Does Calcium Form A Negative Ion Transition metals have a loot of. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. It is not calcium(ii) chloride, because calcium forms only one cation when it forms an ion, and it has a characteristic charge of 2+. This requires a. Does Calcium Form A Negative Ion.
From spmchemistry.blog.onlinetuition.com.my
Formation of Negative Ions SPM Chemistry Does Calcium Form A Negative Ion An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. Transition metals have a loot of. An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. Thus, nonmetals tend to form negative ions. A cation (a positive ion) forms when a neutral atom loses one or. Does Calcium Form A Negative Ion.
From www.numerade.com
draw models to represent the formation of the positive calcium ion and Does Calcium Form A Negative Ion Ca 2+ is the calcium ion. Transition metals have a loot of. Thus, we must have two negative charges to balance the 2+ charge of the calcium ion. An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. A cation (a positive ion) forms when a neutral atom loses one or more electrons. Does Calcium Form A Negative Ion.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Does Calcium Form A Negative Ion An anion (negatively charged ion) forms when one or more electrons are added to a parent atom. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Positively charged ions are called cations, and negatively charged ions are called. Transition metals have a. Does Calcium Form A Negative Ion.
From vdocuments.mx
Warm Up What type of ion does Calcium form? Anion or Cation Does it Does Calcium Form A Negative Ion A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when. Transition metals have a loot of. An ion symbol incorporates the charge of the ion as a superscript on the corresponding elemental symbol. This requires a ratio of one ca 2+ ion to. Does Calcium Form A Negative Ion.