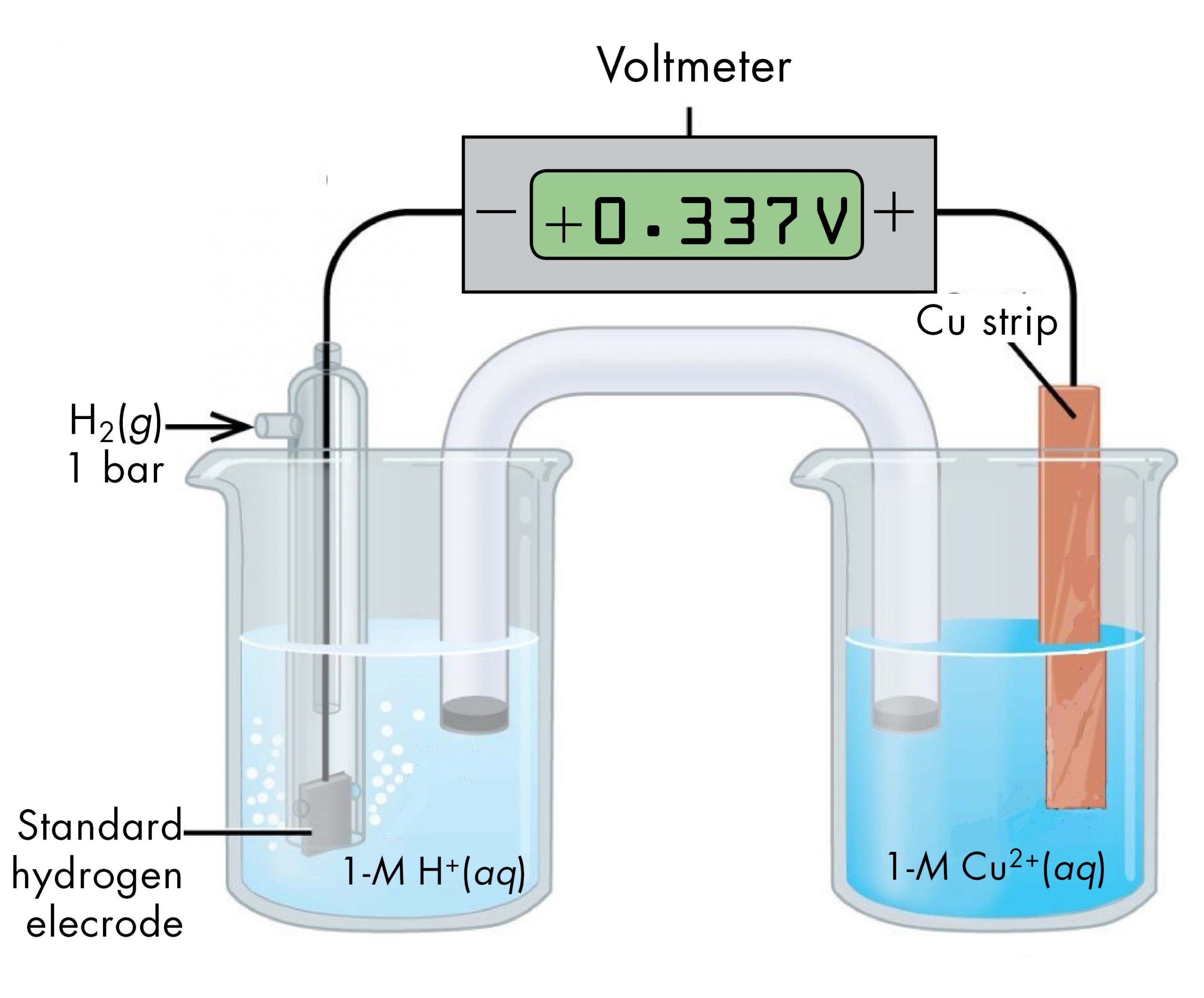
Electrode Potential Hydrogen Half Cell . For example, the standard electrode. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. The structure of the standard hydrogen electrode is. It has a cell potential of 0.00v, measured under standard. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with an acid.
from wisc.pb.unizin.org
The structure of the standard hydrogen electrode is. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with an acid. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. For example, the standard electrode. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. It has a cell potential of 0.00v, measured under standard.
D40.3 Standard HalfCell Potentials Chemistry 109 Fall 2021
Electrode Potential Hydrogen Half Cell The structure of the standard hydrogen electrode is. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with an acid. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. For example, the standard electrode. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The structure of the standard hydrogen electrode is. It has a cell potential of 0.00v, measured under standard.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Electrode Potential Hydrogen Half Cell It has a cell potential of 0.00v, measured under standard. For example, the standard electrode. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with an acid. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. The. Electrode Potential Hydrogen Half Cell.
From www.youtube.com
calculation of half cell potential electrochemistry 2 class 12 Electrode Potential Hydrogen Half Cell It has a cell potential of 0.00v, measured under standard. The structure of the standard hydrogen electrode is. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with. Electrode Potential Hydrogen Half Cell.
From chem.libretexts.org
5.4 Day 39 Voltaic Cells, HalfCell Potentials Chemistry LibreTexts Electrode Potential Hydrogen Half Cell The structure of the standard hydrogen electrode is. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. For. Electrode Potential Hydrogen Half Cell.
From revise.im
Redox and Half Cells Revise.im Electrode Potential Hydrogen Half Cell The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. It has a cell potential of 0.00v, measured under standard. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the. Electrode Potential Hydrogen Half Cell.
From fr.nextews.com
Quel est le potentiel d'électrode? Electrode Potential Hydrogen Half Cell The structure of the standard hydrogen electrode is. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and. Electrode Potential Hydrogen Half Cell.
From www.chemistrystudent.com
Electrochemistry (ALevel) ChemistryStudent Electrode Potential Hydrogen Half Cell It has a cell potential of 0.00v, measured under standard. The structure of the standard hydrogen electrode is. For example, the standard electrode. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard. Electrode Potential Hydrogen Half Cell.
From www.slideserve.com
PPT Chapter 5 Biopotential Electrodes by Michael R. Neuman PowerPoint Electrode Potential Hydrogen Half Cell It has a cell potential of 0.00v, measured under standard. The structure of the standard hydrogen electrode is. For example, the standard electrode. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with an acid. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode. Electrode Potential Hydrogen Half Cell.
From gaskatel.de
Principles of the pHvalue measurement with hydrogen electrodes Gaskatel Electrode Potential Hydrogen Half Cell The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. It has a cell potential of 0.00v, measured under standard. The structure of the standard hydrogen electrode is. The standard hydrogen electrode is constructed so that h 2. Electrode Potential Hydrogen Half Cell.
From chem.libretexts.org
5.4 Day 39 Voltaic Cells, HalfCell Potentials Chemistry LibreTexts Electrode Potential Hydrogen Half Cell The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with an acid. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. For example, the. Electrode Potential Hydrogen Half Cell.
From wisc.pb.unizin.org
D40.3 Standard HalfCell Potentials Chemistry 109 Fall 2021 Electrode Potential Hydrogen Half Cell In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with an acid. It has a cell potential of 0.00v, measured under standard. The structure of the standard hydrogen. Electrode Potential Hydrogen Half Cell.
From psu.pb.unizin.org
17.3 Standard Reduction Potentials Chemistry 112 Chapters 1217 of Electrode Potential Hydrogen Half Cell It has a cell potential of 0.00v, measured under standard. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. For example, the standard electrode. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential. Electrode Potential Hydrogen Half Cell.
From chemistryexplainedpsychokillerblogger.blogspot.com
Chemistry Explained Electrode Potential (Introduction) Electrode Potential Hydrogen Half Cell The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. For example, the standard electrode. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with. Electrode Potential Hydrogen Half Cell.
From www.askiitians.com
Electrode Potential Study Material for IIT JEE askIITians Electrode Potential Hydrogen Half Cell The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with an acid. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. In electrochemistry, the. Electrode Potential Hydrogen Half Cell.
From socratic.org
Two halfcells in a galvanic cell consist of one iron (Fe(s)) electrode Electrode Potential Hydrogen Half Cell In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with an acid. For example, the standard electrode. The emf measured when a metal / metal ion electrode is. Electrode Potential Hydrogen Half Cell.
From www.hanlin.com
CIE A Level Chemistry复习笔记5.3.4 Measuring the Standard Electrode Electrode Potential Hydrogen Half Cell The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with an acid. It has a. Electrode Potential Hydrogen Half Cell.
From saylordotorg.github.io
Describing Electrochemical Cells Electrode Potential Hydrogen Half Cell The structure of the standard hydrogen electrode is. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. It has a cell potential of 0.00v, measured under standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. Electrode Potential Hydrogen Half Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrode Potential Hydrogen Half Cell The structure of the standard hydrogen electrode is. It has a cell potential of 0.00v, measured under standard. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. For example, the standard electrode. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard. Electrode Potential Hydrogen Half Cell.
From www.youtube.com
Standard Half Cell Potentials YouTube Electrode Potential Hydrogen Half Cell The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. The structure of the standard hydrogen electrode is. It. Electrode Potential Hydrogen Half Cell.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Electrode Potential Hydrogen Half Cell It has a cell potential of 0.00v, measured under standard. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. For example, the standard electrode. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential. Electrode Potential Hydrogen Half Cell.
From meteonmendez.blogspot.com
Standard Electrode Potential Table MeteonMendez Electrode Potential Hydrogen Half Cell It has a cell potential of 0.00v, measured under standard. The structure of the standard hydrogen electrode is. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a. Electrode Potential Hydrogen Half Cell.
From app.pandai.org
Standard Electrode Potential Electrode Potential Hydrogen Half Cell The structure of the standard hydrogen electrode is. For example, the standard electrode. It has a cell potential of 0.00v, measured under standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. In electrochemistry, the standard hydrogen. Electrode Potential Hydrogen Half Cell.
From saylordotorg.github.io
Electrochemistry Electrode Potential Hydrogen Half Cell The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with an acid. The structure of the standard hydrogen electrode is. For example, the standard electrode. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as. Electrode Potential Hydrogen Half Cell.
From quizlet.com
ELECTRODE POTENTIAL AND CELL Diagram Quizlet Electrode Potential Hydrogen Half Cell The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with an acid. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. It has a. Electrode Potential Hydrogen Half Cell.
From mmerevise.co.uk
Electrochemical Cells MME Electrode Potential Hydrogen Half Cell The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with an acid. It has a. Electrode Potential Hydrogen Half Cell.
From studylib.net
electrochemistry 2 Electrode Potential Hydrogen Half Cell In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. It has a cell potential of 0.00v, measured under. Electrode Potential Hydrogen Half Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID4491325 Electrode Potential Hydrogen Half Cell The structure of the standard hydrogen electrode is. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with an acid. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of. Electrode Potential Hydrogen Half Cell.
From boisestate.pressbooks.pub
17.3 Standard Reduction Potentials General Chemistry 1 & 2 Electrode Potential Hydrogen Half Cell For example, the standard electrode. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. It has a cell. Electrode Potential Hydrogen Half Cell.
From www.pinterest.jp
Electrochemistry Electrochemistry, Chemistry classroom, Science chemistry Electrode Potential Hydrogen Half Cell In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. The structure of the standard hydrogen electrode is. It has a cell potential of 0.00v, measured under standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. Electrode Potential Hydrogen Half Cell.
From cartoondealer.com
A Standard Hydrogen Electrode (SHE) Is An Electrode That Scientists Use Electrode Potential Hydrogen Half Cell It has a cell potential of 0.00v, measured under standard. For example, the standard electrode. The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with an acid. The structure of the standard hydrogen electrode is. The emf measured when a metal / metal ion electrode is coupled. Electrode Potential Hydrogen Half Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Electrode Potential Hydrogen Half Cell In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. It has a cell potential of 0.00v, measured under standard. The structure of the standard hydrogen electrode is. For example, the standard electrode. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard. Electrode Potential Hydrogen Half Cell.
From question.pandai.org
Standard Electrode Potential Electrode Potential Hydrogen Half Cell For example, the standard electrode. It has a cell potential of 0.00v, measured under standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard hydrogen electrode is constructed so that h 2 gas flows over. Electrode Potential Hydrogen Half Cell.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Electrode Potential Hydrogen Half Cell The standard hydrogen electrode is constructed so that h 2 gas flows over an inert electrode made of platinum, and can interact with an acid. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. In electrochemistry, the. Electrode Potential Hydrogen Half Cell.
From pandai.me
Standard Electrode Potential Electrode Potential Hydrogen Half Cell For example, the standard electrode. It has a cell potential of 0.00v, measured under standard. The structure of the standard hydrogen electrode is. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard hydrogen electrode is. Electrode Potential Hydrogen Half Cell.
From slideplayer.com
Electrochemical Cells ppt download Electrode Potential Hydrogen Half Cell It has a cell potential of 0.00v, measured under standard. In electrochemistry, the standard hydrogen electrode (abbreviated she), is a redox electrode which forms the basis of the thermodynamic. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal. Electrode Potential Hydrogen Half Cell.
From monomole.com
Standard hydrogen electrode Mono Mole Electrode Potential Hydrogen Half Cell The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The structure of the standard hydrogen electrode is. For example, the standard electrode. It has a cell potential of 0.00v, measured under standard. The standard hydrogen electrode is. Electrode Potential Hydrogen Half Cell.