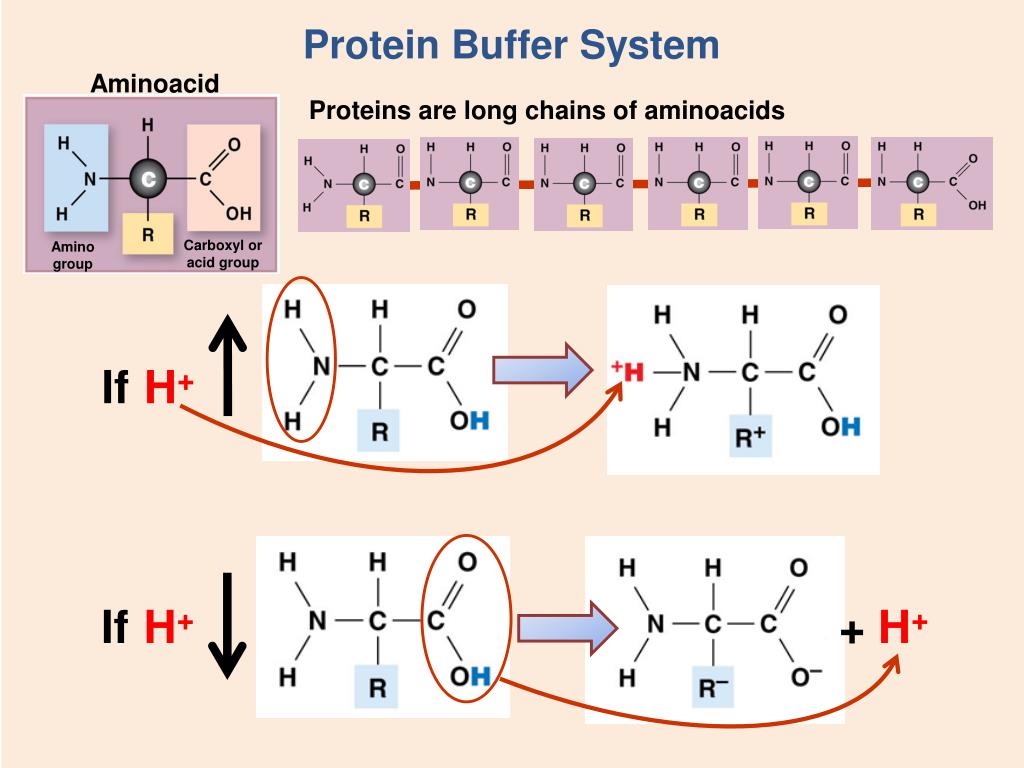
Proteins Are Effective Buffers Because . Buffering is by the imidazole group of the histidine residues. The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. The protein hemoglobin makes an excellent buffer. Bicarbonate is an effective buffer because it is: Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Protein buffers in blood include haemoglobin (150g/l) and plasma proteins (70g/l). Co 2 can be adjusted by changing. It can bind to small amounts of acid in the blood, helping to remove that acid before it. Buffer solutions consist of a weak acid and its conjugate base.
from www.slideserve.com
Buffering is by the imidazole group of the histidine residues. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The protein hemoglobin makes an excellent buffer. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. It can bind to small amounts of acid in the blood, helping to remove that acid before it. Co 2 can be adjusted by changing. Bicarbonate is an effective buffer because it is: Buffer solutions consist of a weak acid and its conjugate base. Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations.
PPT Lesson 22 PowerPoint Presentation, free download ID2821906
Proteins Are Effective Buffers Because Co 2 can be adjusted by changing. Co 2 can be adjusted by changing. Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations. Buffering is by the imidazole group of the histidine residues. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Bicarbonate is an effective buffer because it is: The protein hemoglobin makes an excellent buffer. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Protein buffers in blood include haemoglobin (150g/l) and plasma proteins (70g/l). It can bind to small amounts of acid in the blood, helping to remove that acid before it. Buffer solutions consist of a weak acid and its conjugate base. The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID6870820 Proteins Are Effective Buffers Because Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations. Buffering is by the imidazole group of the histidine residues. Protein buffers in blood include haemoglobin (150g/l) and plasma proteins (70g/l). The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. Proteins Are Effective Buffers Because.
From jpharmsci.org
Role of Buffers in Protein Formulations Journal of Pharmaceutical Proteins Are Effective Buffers Because A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. The protein hemoglobin makes. Proteins Are Effective Buffers Because.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Proteins Are Effective Buffers Because Protein buffers in blood include haemoglobin (150g/l) and plasma proteins (70g/l). Buffer solutions consist of a weak acid and its conjugate base. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Buffering is by the imidazole group of the histidine residues. Proteins in the body contain side chains that can. Proteins Are Effective Buffers Because.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID Proteins Are Effective Buffers Because Buffer solutions consist of a weak acid and its conjugate base. Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations. Protein buffers in blood include haemoglobin (150g/l) and plasma proteins (70g/l). The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and. Proteins Are Effective Buffers Because.
From slideplayer.com
Advanced Physiology (part 6, Acidbase Balance) ppt download Proteins Are Effective Buffers Because Bicarbonate is an effective buffer because it is: Protein buffers in blood include haemoglobin (150g/l) and plasma proteins (70g/l). The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. The protein hemoglobin makes an excellent buffer. Buffer solutions consist of a weak acid and its conjugate base. It can bind to. Proteins Are Effective Buffers Because.
From theoreticaldoctor.com
Proteins Properties of Protein The Theoretical Doctor Proteins Are Effective Buffers Because A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The protein hemoglobin makes an excellent buffer. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or. Proteins Are Effective Buffers Because.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID2240409 Proteins Are Effective Buffers Because The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations. The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. It can. Proteins Are Effective Buffers Because.
From www.stellarscientific.com
Bottle of EasyPrepProtein Extraction Buffer For Extracting Proteins Proteins Are Effective Buffers Because Protein buffers in blood include haemoglobin (150g/l) and plasma proteins (70g/l). Co 2 can be adjusted by changing. Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations. The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. Bicarbonate is an. Proteins Are Effective Buffers Because.
From www.slideserve.com
PPT Acidbase and buffer PowerPoint Presentation, free download ID Proteins Are Effective Buffers Because A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffering is by the imidazole group of the histidine residues. Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations. Buffer solutions consist of a weak acid and its conjugate. Proteins Are Effective Buffers Because.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 Proteins Are Effective Buffers Because The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Bicarbonate is an effective buffer because it is: Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations. Buffer solutions consist of a weak acid and its conjugate base. The. Proteins Are Effective Buffers Because.
From www.slideserve.com
PPT AcidBase Balance PowerPoint Presentation, free download ID2821926 Proteins Are Effective Buffers Because The protein hemoglobin makes an excellent buffer. The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Bicarbonate is an effective buffer because it is: It can bind to small amounts of acid. Proteins Are Effective Buffers Because.
From www.slideserve.com
PPT Lesson 22 PowerPoint Presentation, free download ID2821906 Proteins Are Effective Buffers Because Buffer solutions consist of a weak acid and its conjugate base. Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations. Co 2 can be adjusted by changing. The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. Bicarbonate is an. Proteins Are Effective Buffers Because.
From goldbio.com
All About the Composition of Protein Purification Buffers and Why It Proteins Are Effective Buffers Because Buffer solutions consist of a weak acid and its conjugate base. Co 2 can be adjusted by changing. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It can bind to small amounts of acid in the blood, helping to remove that acid before it. The protein hemoglobin makes an. Proteins Are Effective Buffers Because.
From www.researchgate.net
Protein extraction buffer compositions. Download Scientific Diagram Proteins Are Effective Buffers Because The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations. Protein buffers in blood include haemoglobin (150g/l) and plasma proteins (70g/l). Bicarbonate is an effective buffer because it is: It. Proteins Are Effective Buffers Because.
From www.slideserve.com
PPT (Renal Physiology 10) AcidBase Balance 2 Buffers System Proteins Are Effective Buffers Because The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. The protein hemoglobin makes an excellent buffer. Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations. Protein buffers in blood include haemoglobin (150g/l) and plasma proteins (70g/l). Bicarbonate is an. Proteins Are Effective Buffers Because.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts Proteins Are Effective Buffers Because Buffer solutions consist of a weak acid and its conjugate base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffering is by the imidazole group of the histidine residues. The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. The most. Proteins Are Effective Buffers Because.
From goldbio.com
All About the Composition of Protein Purification Buffers and Why It Proteins Are Effective Buffers Because The protein hemoglobin makes an excellent buffer. It can bind to small amounts of acid in the blood, helping to remove that acid before it. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Protein buffers in blood include haemoglobin (150g/l) and plasma proteins (70g/l). The ability of buffers to. Proteins Are Effective Buffers Because.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and Proteins Are Effective Buffers Because Co 2 can be adjusted by changing. Buffering is by the imidazole group of the histidine residues. Bicarbonate is an effective buffer because it is: The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. Buffer solutions consist of a weak acid and its conjugate base. Protein buffers in blood include haemoglobin. Proteins Are Effective Buffers Because.
From www.slideserve.com
PPT ABC’s of pH PowerPoint Presentation, free download ID6305928 Proteins Are Effective Buffers Because Bicarbonate is an effective buffer because it is: Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Co 2 can be adjusted by changing. The ability of buffers to. Proteins Are Effective Buffers Because.
From www.slideserve.com
PPT Acid and Base Balance PowerPoint Presentation, free download ID Proteins Are Effective Buffers Because It can bind to small amounts of acid in the blood, helping to remove that acid before it. The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations. Buffer solutions consist. Proteins Are Effective Buffers Because.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation Proteins Are Effective Buffers Because It can bind to small amounts of acid in the blood, helping to remove that acid before it. Co 2 can be adjusted by changing. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Protein buffers in blood include haemoglobin (150g/l) and plasma proteins (70g/l). The ability of buffers to. Proteins Are Effective Buffers Because.
From www.chegg.com
Solved Proteins are good buffers in the interstitial fluid. Proteins Are Effective Buffers Because The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. Bicarbonate is an effective buffer because it is: Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations. The protein hemoglobin makes an excellent buffer. Protein buffers in blood include haemoglobin. Proteins Are Effective Buffers Because.
From en.ppt-online.org
Solutions. Acidbase equilibrium in biological systems online Proteins Are Effective Buffers Because Bicarbonate is an effective buffer because it is: The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. Co 2 can be adjusted by changing. Buffering is by the imidazole group of the. Proteins Are Effective Buffers Because.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Proteins Are Effective Buffers Because The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. It can bind to small amounts of acid in the blood, helping to remove that acid before it. Buffering is by the imidazole group of the histidine residues. The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen. Proteins Are Effective Buffers Because.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Proteins Are Effective Buffers Because Buffer solutions consist of a weak acid and its conjugate base. Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The ability of buffers to stabilize therapeutic proteins whether. Proteins Are Effective Buffers Because.
From www.slideserve.com
PPT ACIDBASE BALANCE PowerPoint Presentation, free download ID6579634 Proteins Are Effective Buffers Because It can bind to small amounts of acid in the blood, helping to remove that acid before it. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Buffer solutions consist of a weak acid and its conjugate base. Proteins in the body contain side chains that can either release or. Proteins Are Effective Buffers Because.
From www.slideshare.net
Ch 26 Proteins Are Effective Buffers Because Co 2 can be adjusted by changing. The protein hemoglobin makes an excellent buffer. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. Buffer solutions consist of a weak acid and its. Proteins Are Effective Buffers Because.
From www.slideserve.com
PPT Structure & Classification of Proteins PowerPoint Presentation Proteins Are Effective Buffers Because The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Protein buffers in blood include haemoglobin (150g/l) and plasma proteins (70g/l). The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. Buffer solutions consist of a weak acid and its conjugate base. A. Proteins Are Effective Buffers Because.
From www.youtube.com
Protein Buffering System YouTube Proteins Are Effective Buffers Because It can bind to small amounts of acid in the blood, helping to remove that acid before it. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Co 2 can be adjusted by changing. The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the. Proteins Are Effective Buffers Because.
From www.slideshare.net
Buffer system Proteins Are Effective Buffers Because The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The protein hemoglobin makes an excellent buffer. Protein buffers in blood include haemoglobin (150g/l) and plasma proteins (70g/l). It can bind to. Proteins Are Effective Buffers Because.
From deporecipe.co
10x Protein Running Buffer Recipe Deporecipe.co Proteins Are Effective Buffers Because Bicarbonate is an effective buffer because it is: Co 2 can be adjusted by changing. The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. Buffering is by the imidazole group of the histidine residues. A buffer is a solution that can resist ph change upon the addition of an acidic or. Proteins Are Effective Buffers Because.
From slideplayer.com
Acid base balance Dr. S. Parthasarathy MD., DA., DNB, MD (Acu), Dip Proteins Are Effective Buffers Because A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Buffering is by the imidazole group of the histidine residues. Co 2 can be adjusted by changing. Proteins in the body contain. Proteins Are Effective Buffers Because.
From www.sliderbase.com
Protein Buffers Proteins Are Effective Buffers Because Bicarbonate is an effective buffer because it is: The ability of buffers to stabilize therapeutic proteins whether in liquid formulations, frozen solutions, or the solid state. Protein buffers in blood include haemoglobin (150g/l) and plasma proteins (70g/l). Buffer solutions consist of a weak acid and its conjugate base. It can bind to small amounts of acid in the blood, helping. Proteins Are Effective Buffers Because.
From www.researchgate.net
The used buffers for native (A) and denaturing (B) methods in protein Proteins Are Effective Buffers Because The protein hemoglobin makes an excellent buffer. It can bind to small amounts of acid in the blood, helping to remove that acid before it. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling. Proteins Are Effective Buffers Because.
From www.stellarscientific.com
Bottle of EasyPrepProtein Extraction Buffer For Extracting Proteins Proteins Are Effective Buffers Because It can bind to small amounts of acid in the blood, helping to remove that acid before it. Proteins in the body contain side chains that can either release or bind hydrogen ions, enabling them to stabilize ph fluctuations. Bicarbonate is an effective buffer because it is: Co 2 can be adjusted by changing. The ability of buffers to stabilize. Proteins Are Effective Buffers Because.