Conclusion For Distillation Experiment . Simple distillations are used in. The data collected in the. What is a simple distillation experiment? In this method, the mixture to be. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. To separate a mixture of two miscible liquids. The experiment was successful in separate e thanol from the mixture with water by simple and fractional distillation. We utilize the difference in boiling points of liquids. Conclusion the purpose of this fractional distillation lab was to separate mixtures of liquids that have similar boiling points,. As outlined in the above table and graph the temperature spiked when the volumed first. Simple distillation separates components from their liquid mixtures based on their boiling point differences. Distillation is an effective method of separating the constituent parts of a mixture. Distillation is a purification technique for a liquid or a mixture of liquids. The goal of this experiment was to extract citrus essential oils from the peels of citrus fruits through simple distillation.
from www.slideserve.com
We utilize the difference in boiling points of liquids. Simple distillation separates components from their liquid mixtures based on their boiling point differences. Distillation is an effective method of separating the constituent parts of a mixture. In this method, the mixture to be. To separate a mixture of two miscible liquids. Distillation is a purification technique for a liquid or a mixture of liquids. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. As outlined in the above table and graph the temperature spiked when the volumed first. The experiment was successful in separate e thanol from the mixture with water by simple and fractional distillation. The data collected in the.
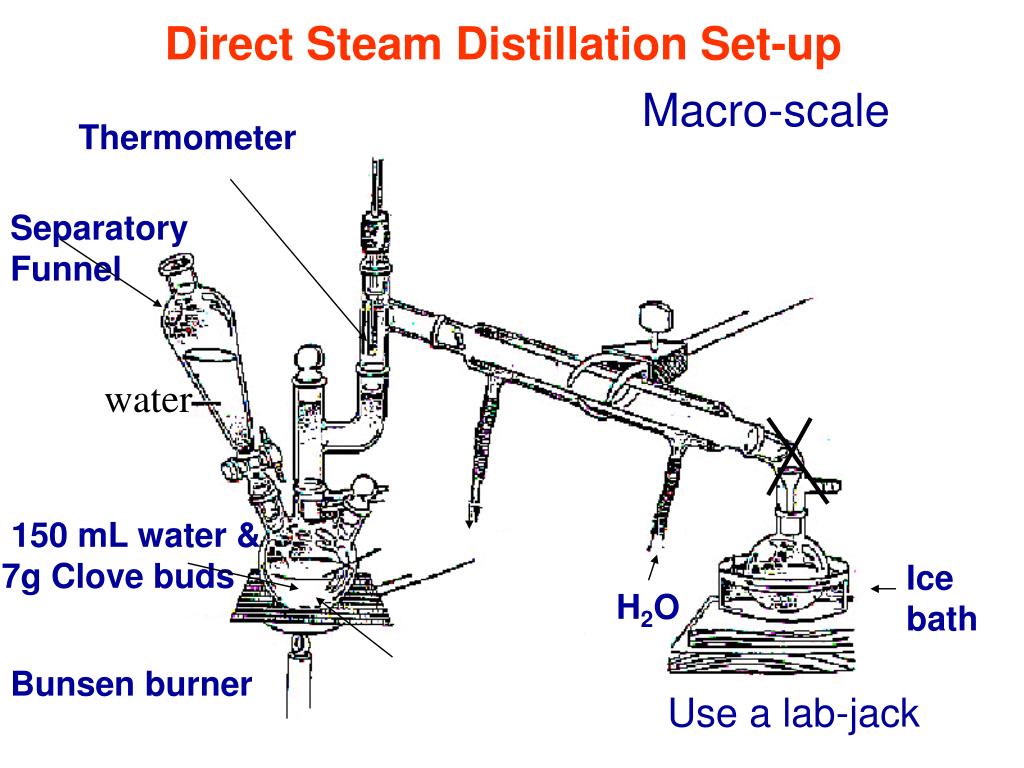
PPT Today Conclusion of Distillation/GC Introduction to Exp.4 Steam
Conclusion For Distillation Experiment As outlined in the above table and graph the temperature spiked when the volumed first. What is a simple distillation experiment? In this method, the mixture to be. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Distillation is an effective method of separating the constituent parts of a mixture. Simple distillations are used in. Distillation is a purification technique for a liquid or a mixture of liquids. The experiment was successful in separate e thanol from the mixture with water by simple and fractional distillation. As outlined in the above table and graph the temperature spiked when the volumed first. To separate a mixture of two miscible liquids. Conclusion the purpose of this fractional distillation lab was to separate mixtures of liquids that have similar boiling points,. Simple distillation separates components from their liquid mixtures based on their boiling point differences. The data collected in the. The goal of this experiment was to extract citrus essential oils from the peels of citrus fruits through simple distillation. We utilize the difference in boiling points of liquids.
From www.numerade.com
SOLVED A student did a distillation experiment using 40 aqueous Conclusion For Distillation Experiment As outlined in the above table and graph the temperature spiked when the volumed first. The goal of this experiment was to extract citrus essential oils from the peels of citrus fruits through simple distillation. To separate a mixture of two miscible liquids. Simple distillations are used in. Conclusion the purpose of this fractional distillation lab was to separate mixtures. Conclusion For Distillation Experiment.
From www.slideserve.com
PPT Simple Distillation PowerPoint Presentation, free download ID Conclusion For Distillation Experiment As outlined in the above table and graph the temperature spiked when the volumed first. Distillation is an effective method of separating the constituent parts of a mixture. The data collected in the. Conclusion the purpose of this fractional distillation lab was to separate mixtures of liquids that have similar boiling points,. Distillation is a purification method for liquids, and. Conclusion For Distillation Experiment.
From www.docsity.com
Simple and fractional distillation lab report Study Guides, Projects Conclusion For Distillation Experiment Conclusion the purpose of this fractional distillation lab was to separate mixtures of liquids that have similar boiling points,. To separate a mixture of two miscible liquids. Distillation is an effective method of separating the constituent parts of a mixture. The data collected in the. Distillation is a purification technique for a liquid or a mixture of liquids. The experiment. Conclusion For Distillation Experiment.
From lugomoni.wixsite.com
Conclusion distillation Conclusion For Distillation Experiment Conclusion the purpose of this fractional distillation lab was to separate mixtures of liquids that have similar boiling points,. The experiment was successful in separate e thanol from the mixture with water by simple and fractional distillation. The goal of this experiment was to extract citrus essential oils from the peels of citrus fruits through simple distillation. Simple distillations are. Conclusion For Distillation Experiment.
From www.chegg.com
Solved Steam Distillation Lab These are the conclusion Conclusion For Distillation Experiment Distillation is a purification technique for a liquid or a mixture of liquids. The experiment was successful in separate e thanol from the mixture with water by simple and fractional distillation. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. We utilize the difference in boiling points of. Conclusion For Distillation Experiment.
From www.vernier.com
Fractional Distillation > Experiment 8 from Chemistry with Vernier Conclusion For Distillation Experiment As outlined in the above table and graph the temperature spiked when the volumed first. The goal of this experiment was to extract citrus essential oils from the peels of citrus fruits through simple distillation. In this method, the mixture to be. To separate a mixture of two miscible liquids. We utilize the difference in boiling points of liquids. The. Conclusion For Distillation Experiment.
From phdessay.com
Fractional Distillation Experiment (300 Words) Conclusion For Distillation Experiment Distillation is a purification technique for a liquid or a mixture of liquids. In this method, the mixture to be. What is a simple distillation experiment? The experiment was successful in separate e thanol from the mixture with water by simple and fractional distillation. As outlined in the above table and graph the temperature spiked when the volumed first. Simple. Conclusion For Distillation Experiment.
From www.studocu.com
Separation of Liquids Fractional Distillation and Gas Chromatography Conclusion For Distillation Experiment We utilize the difference in boiling points of liquids. Distillation is a purification technique for a liquid or a mixture of liquids. Conclusion the purpose of this fractional distillation lab was to separate mixtures of liquids that have similar boiling points,. To separate a mixture of two miscible liquids. Simple distillations are used in. Simple distillation separates components from their. Conclusion For Distillation Experiment.
From www.researchgate.net
(PDF) simple distillation Conclusion For Distillation Experiment Simple distillations are used in. The data collected in the. As outlined in the above table and graph the temperature spiked when the volumed first. In this method, the mixture to be. To separate a mixture of two miscible liquids. We utilize the difference in boiling points of liquids. Conclusion the purpose of this fractional distillation lab was to separate. Conclusion For Distillation Experiment.
From www.slideserve.com
PPT DISTILLATION PowerPoint Presentation, free download ID2250229 Conclusion For Distillation Experiment Distillation is an effective method of separating the constituent parts of a mixture. The data collected in the. The goal of this experiment was to extract citrus essential oils from the peels of citrus fruits through simple distillation. Distillation is a purification technique for a liquid or a mixture of liquids. To separate a mixture of two miscible liquids. As. Conclusion For Distillation Experiment.
From www.keetoncustomgolf.com
1 Distillation lab report. Homework Help Sites. Conclusion For Distillation Experiment Conclusion the purpose of this fractional distillation lab was to separate mixtures of liquids that have similar boiling points,. Distillation is an effective method of separating the constituent parts of a mixture. As outlined in the above table and graph the temperature spiked when the volumed first. Distillation is a purification technique for a liquid or a mixture of liquids.. Conclusion For Distillation Experiment.
From www.chegg.com
Solved Conclusion In the conclusion the aim of experiment Conclusion For Distillation Experiment The experiment was successful in separate e thanol from the mixture with water by simple and fractional distillation. As outlined in the above table and graph the temperature spiked when the volumed first. The data collected in the. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. In. Conclusion For Distillation Experiment.
From www.circuitdiagram.co
Schematic Diagram Of Simple Distillation Experiment Circuit Diagram Conclusion For Distillation Experiment Distillation is an effective method of separating the constituent parts of a mixture. Distillation is a purification technique for a liquid or a mixture of liquids. In this method, the mixture to be. We utilize the difference in boiling points of liquids. The goal of this experiment was to extract citrus essential oils from the peels of citrus fruits through. Conclusion For Distillation Experiment.
From www.chemicals.co.uk
What is the Distillation Process? The Chemistry Blog Conclusion For Distillation Experiment The data collected in the. Distillation is an effective method of separating the constituent parts of a mixture. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. We utilize the difference in boiling points of liquids. The experiment was successful in separate e thanol from the mixture with. Conclusion For Distillation Experiment.
From www.studocu.com
Distillation Lab Conclusion Discussion, sources of error, and Conclusion For Distillation Experiment The goal of this experiment was to extract citrus essential oils from the peels of citrus fruits through simple distillation. As outlined in the above table and graph the temperature spiked when the volumed first. Simple distillations are used in. We utilize the difference in boiling points of liquids. Simple distillation separates components from their liquid mixtures based on their. Conclusion For Distillation Experiment.
From www.slideserve.com
PPT Today Conclusion of Distillation/GC Introduction to Exp.4 Steam Conclusion For Distillation Experiment Conclusion the purpose of this fractional distillation lab was to separate mixtures of liquids that have similar boiling points,. What is a simple distillation experiment? We utilize the difference in boiling points of liquids. In this method, the mixture to be. The goal of this experiment was to extract citrus essential oils from the peels of citrus fruits through simple. Conclusion For Distillation Experiment.
From www.slideserve.com
PPT Simple distillation and fractional distillation PowerPoint Conclusion For Distillation Experiment To separate a mixture of two miscible liquids. What is a simple distillation experiment? As outlined in the above table and graph the temperature spiked when the volumed first. Simple distillation separates components from their liquid mixtures based on their boiling point differences. The goal of this experiment was to extract citrus essential oils from the peels of citrus fruits. Conclusion For Distillation Experiment.
From www.chegg.com
Solved EXPERIMENT 6 Simple and Fractional Distillation Conclusion For Distillation Experiment What is a simple distillation experiment? We utilize the difference in boiling points of liquids. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Distillation is an effective method of separating the constituent parts of a mixture. The experiment was successful in separate e thanol from the mixture. Conclusion For Distillation Experiment.
From www.numerade.com
SOLVED A student did a distillation experiment using 40 aqueous Conclusion For Distillation Experiment As outlined in the above table and graph the temperature spiked when the volumed first. Simple distillations are used in. The goal of this experiment was to extract citrus essential oils from the peels of citrus fruits through simple distillation. To separate a mixture of two miscible liquids. In this method, the mixture to be. Distillation is an effective method. Conclusion For Distillation Experiment.
From www.numerade.com
SOLVED A student did a distillation experiment using 40 aqueous Conclusion For Distillation Experiment The goal of this experiment was to extract citrus essential oils from the peels of citrus fruits through simple distillation. What is a simple distillation experiment? As outlined in the above table and graph the temperature spiked when the volumed first. Conclusion the purpose of this fractional distillation lab was to separate mixtures of liquids that have similar boiling points,.. Conclusion For Distillation Experiment.
From studylib.net
Experiment 2. Separation of Liquids by Distillation. Conclusion For Distillation Experiment The goal of this experiment was to extract citrus essential oils from the peels of citrus fruits through simple distillation. As outlined in the above table and graph the temperature spiked when the volumed first. What is a simple distillation experiment? Simple distillation separates components from their liquid mixtures based on their boiling point differences. Distillation is a purification technique. Conclusion For Distillation Experiment.
From www.scribd.com
Nates Conclusion PDF Distillation Experiment Conclusion For Distillation Experiment To separate a mixture of two miscible liquids. What is a simple distillation experiment? As outlined in the above table and graph the temperature spiked when the volumed first. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Simple distillation separates components from their liquid mixtures based on. Conclusion For Distillation Experiment.
From www.slideserve.com
PPT Today Conclusion of Distillation/GC Introduction to Exp.4 Steam Conclusion For Distillation Experiment Distillation is a purification technique for a liquid or a mixture of liquids. To separate a mixture of two miscible liquids. Distillation is an effective method of separating the constituent parts of a mixture. The goal of this experiment was to extract citrus essential oils from the peels of citrus fruits through simple distillation. The experiment was successful in separate. Conclusion For Distillation Experiment.
From www.alamy.com
Science experiment with distillation illustration Stock Vector Image Conclusion For Distillation Experiment In this method, the mixture to be. What is a simple distillation experiment? Distillation is a purification technique for a liquid or a mixture of liquids. The experiment was successful in separate e thanol from the mixture with water by simple and fractional distillation. Simple distillation separates components from their liquid mixtures based on their boiling point differences. The data. Conclusion For Distillation Experiment.
From www.youtube.com
Introduction to Fractional distillation Distillation procedure Home Conclusion For Distillation Experiment Simple distillation separates components from their liquid mixtures based on their boiling point differences. Distillation is an effective method of separating the constituent parts of a mixture. We utilize the difference in boiling points of liquids. The goal of this experiment was to extract citrus essential oils from the peels of citrus fruits through simple distillation. Simple distillations are used. Conclusion For Distillation Experiment.
From www.slideshare.net
Simple distillation Conclusion For Distillation Experiment We utilize the difference in boiling points of liquids. Conclusion the purpose of this fractional distillation lab was to separate mixtures of liquids that have similar boiling points,. In this method, the mixture to be. Simple distillation separates components from their liquid mixtures based on their boiling point differences. As outlined in the above table and graph the temperature spiked. Conclusion For Distillation Experiment.
From www.chegg.com
Solved STEAM DISTILLATION The purpose of this experiment is Conclusion For Distillation Experiment As outlined in the above table and graph the temperature spiked when the volumed first. Conclusion the purpose of this fractional distillation lab was to separate mixtures of liquids that have similar boiling points,. Distillation is an effective method of separating the constituent parts of a mixture. We utilize the difference in boiling points of liquids. Distillation is a purification. Conclusion For Distillation Experiment.
From paperap.com
Distillation Experiment Conclusion Report Conclusion Essay Example Conclusion For Distillation Experiment The goal of this experiment was to extract citrus essential oils from the peels of citrus fruits through simple distillation. To separate a mixture of two miscible liquids. Simple distillation separates components from their liquid mixtures based on their boiling point differences. We utilize the difference in boiling points of liquids. What is a simple distillation experiment? Distillation is a. Conclusion For Distillation Experiment.
From studylib.net
Steam Distillation Experiment Conclusion For Distillation Experiment Distillation is an effective method of separating the constituent parts of a mixture. We utilize the difference in boiling points of liquids. The experiment was successful in separate e thanol from the mixture with water by simple and fractional distillation. In this method, the mixture to be. Simple distillations are used in. The data collected in the. Conclusion the purpose. Conclusion For Distillation Experiment.
From www.youtube.com
ORGANIC CHEMISTRY EXPERIMENT 3 Distillation Technique YouTube Conclusion For Distillation Experiment Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Simple distillation separates components from their liquid mixtures based on their boiling point differences. We utilize the difference in boiling points of liquids. Distillation is an effective method of separating the constituent parts of a mixture. What is a. Conclusion For Distillation Experiment.
From paperap.com
Simple Distillation Lab Report Report Conclusion Essay Example Conclusion For Distillation Experiment Simple distillations are used in. Simple distillation separates components from their liquid mixtures based on their boiling point differences. To separate a mixture of two miscible liquids. We utilize the difference in boiling points of liquids. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. The data collected. Conclusion For Distillation Experiment.
From www.chegg.com
Solved Distillation Experiment 1. Shown below is boiling Conclusion For Distillation Experiment The experiment was successful in separate e thanol from the mixture with water by simple and fractional distillation. Simple distillation separates components from their liquid mixtures based on their boiling point differences. Conclusion the purpose of this fractional distillation lab was to separate mixtures of liquids that have similar boiling points,. Simple distillations are used in. To separate a mixture. Conclusion For Distillation Experiment.
From www.slideserve.com
PPT Simple distillation and fractional distillation PowerPoint Conclusion For Distillation Experiment As outlined in the above table and graph the temperature spiked when the volumed first. The data collected in the. Simple distillations are used in. In this method, the mixture to be. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Simple distillation separates components from their liquid. Conclusion For Distillation Experiment.
From www.studocu.com
Notebook+Distillation Experiment Distillation Overview In this Conclusion For Distillation Experiment Conclusion the purpose of this fractional distillation lab was to separate mixtures of liquids that have similar boiling points,. What is a simple distillation experiment? Distillation is a purification technique for a liquid or a mixture of liquids. The experiment was successful in separate e thanol from the mixture with water by simple and fractional distillation. Distillation is an effective. Conclusion For Distillation Experiment.
From fyoyrnvmd.blob.core.windows.net
Distillation Conclusion at Maryann Kravitz blog Conclusion For Distillation Experiment Simple distillation separates components from their liquid mixtures based on their boiling point differences. As outlined in the above table and graph the temperature spiked when the volumed first. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. The data collected in the. Simple distillations are used in.. Conclusion For Distillation Experiment.