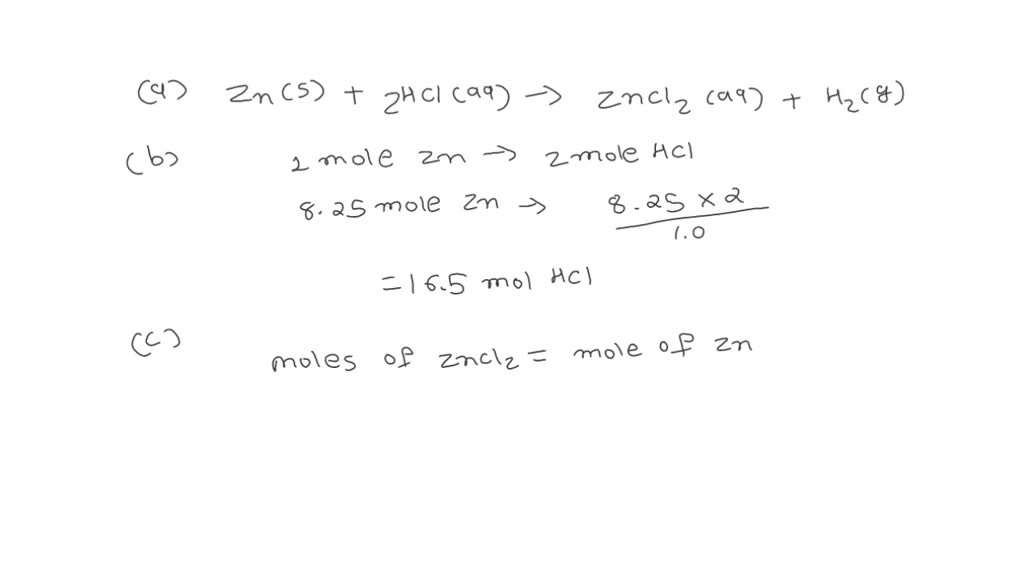Zinc And Hydrochloric Acid Complete Ionic Equation . enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. See the properties and examples of zinc and its interactions with other substances. learn how to balance the equation for the reaction of zinc with hydrochloric acid, a single displacement (substitution). there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. The experiment compares reactivity of three. In the process, hydrogen gas is produced. Zinc is oxidized by hydrochloric acid to form zinc chloride. this experiment includes the reaction of zinc with hydrochloric acid.
from www.numerade.com
learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. See the properties and examples of zinc and its interactions with other substances. Zinc is oxidized by hydrochloric acid to form zinc chloride. this experiment includes the reaction of zinc with hydrochloric acid. The experiment compares reactivity of three. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. learn how to balance the equation for the reaction of zinc with hydrochloric acid, a single displacement (substitution). there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). In the process, hydrogen gas is produced.
SOLVED Consider the reaction of zinc metal with hydrochloric acid, HCl
Zinc And Hydrochloric Acid Complete Ionic Equation learn how to balance the equation for the reaction of zinc with hydrochloric acid, a single displacement (substitution). In the process, hydrogen gas is produced. See the properties and examples of zinc and its interactions with other substances. there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). Zinc is oxidized by hydrochloric acid to form zinc chloride. learn how to balance the equation for the reaction of zinc with hydrochloric acid, a single displacement (substitution). learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. The experiment compares reactivity of three. this experiment includes the reaction of zinc with hydrochloric acid.
From www.numerade.com
SOLVED The equation below shows the reaction of zinc with hydrochloric Zinc And Hydrochloric Acid Complete Ionic Equation The experiment compares reactivity of three. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. See the properties and examples of zinc and its interactions with other substances. learn how to. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.showme.com
Molecular, Complete Ionic, and Net Ionic Equations Science, Chemistry Zinc And Hydrochloric Acid Complete Ionic Equation The experiment compares reactivity of three. this experiment includes the reaction of zinc with hydrochloric acid. In the process, hydrogen gas is produced. Zinc is oxidized by hydrochloric acid to form zinc chloride. learn how to balance the equation for the reaction of zinc with hydrochloric acid, a single displacement (substitution). enter an ionic equation and get. Zinc And Hydrochloric Acid Complete Ionic Equation.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc And Hydrochloric Acid Complete Ionic Equation there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). learn how to balance the equation for the reaction of zinc with hydrochloric acid, a single displacement (substitution). enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions. Zinc And Hydrochloric Acid Complete Ionic Equation.
From dxopfgxpq.blob.core.windows.net
Zinc And Hydrochloric Acid Reaction Explained at John Crawford blog Zinc And Hydrochloric Acid Complete Ionic Equation there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). The experiment compares reactivity of three. this experiment includes the reaction of zinc with hydrochloric acid. See the properties and examples of zinc and its interactions with other substances. In the process, hydrogen gas is. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.solutionspile.com
[Solved] Zinc reacts with hydrochloric acid according to Zinc And Hydrochloric Acid Complete Ionic Equation there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). The experiment compares reactivity of three. Zinc is oxidized by hydrochloric acid to form zinc chloride. See the properties and examples of zinc and its interactions with other substances. learn how to balance the equation. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.slideserve.com
PPT Net Ionic Equation PowerPoint Presentation, free download ID Zinc And Hydrochloric Acid Complete Ionic Equation learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. In the process, hydrogen gas is produced. Zinc is oxidized by hydrochloric acid to form zinc chloride. there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). . Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.tessshebaylo.com
Write An Equation Showing The Ionization Of Hydrochloric Acid In Water Zinc And Hydrochloric Acid Complete Ionic Equation learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. this experiment includes the reaction of zinc with hydrochloric acid. learn how to balance the equation for the reaction of zinc with hydrochloric acid, a single displacement (substitution). Zinc is oxidized by hydrochloric acid to form zinc chloride. The experiment. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.nagwa.com
Question Video Describing the Correct Symbol Equation for the Reaction Zinc And Hydrochloric Acid Complete Ionic Equation this experiment includes the reaction of zinc with hydrochloric acid. Zinc is oxidized by hydrochloric acid to form zinc chloride. In the process, hydrogen gas is produced. there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). learn how to balance the equation for. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.numerade.com
SOLVED Consider the reaction of zinc metal with hydrochloric acid, HCl Zinc And Hydrochloric Acid Complete Ionic Equation enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. The experiment compares reactivity of three. Zinc is oxidized by hydrochloric acid to form zinc chloride. In the process, hydrogen gas is produced.. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid Zinc And Hydrochloric Acid Complete Ionic Equation Zinc is oxidized by hydrochloric acid to form zinc chloride. In the process, hydrogen gas is produced. learn how to balance the equation for the reaction of zinc with hydrochloric acid, a single displacement (substitution). The experiment compares reactivity of three. See the properties and examples of zinc and its interactions with other substances. enter an ionic equation. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube Zinc And Hydrochloric Acid Complete Ionic Equation this experiment includes the reaction of zinc with hydrochloric acid. The experiment compares reactivity of three. See the properties and examples of zinc and its interactions with other substances. In the process, hydrogen gas is produced. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. enter an ionic equation. Zinc And Hydrochloric Acid Complete Ionic Equation.
From slideplayer.com
Chemical Reactions & Equations ppt download Zinc And Hydrochloric Acid Complete Ionic Equation The experiment compares reactivity of three. In the process, hydrogen gas is produced. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). learn how to balance. Zinc And Hydrochloric Acid Complete Ionic Equation.
From dxoygaprp.blob.core.windows.net
What Is Molecular And Ionic at Patricia Ledbetter blog Zinc And Hydrochloric Acid Complete Ionic Equation In the process, hydrogen gas is produced. learn how to balance the equation for the reaction of zinc with hydrochloric acid, a single displacement (substitution). there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). Zinc is oxidized by hydrochloric acid to form zinc chloride.. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc And Hydrochloric Acid Complete Ionic Equation learn how to balance the equation for the reaction of zinc with hydrochloric acid, a single displacement (substitution). In the process, hydrogen gas is produced. The experiment compares reactivity of three. there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). Zinc is oxidized by. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.chegg.com
Solved Write the net ionic equation for the reaction between Zinc And Hydrochloric Acid Complete Ionic Equation The experiment compares reactivity of three. this experiment includes the reaction of zinc with hydrochloric acid. there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). See the properties and examples of zinc and its interactions with other substances. Zinc is oxidized by hydrochloric acid. Zinc And Hydrochloric Acid Complete Ionic Equation.
From thewonderofscience.com
Reaction of zinc and hydrochloric acid (NY) — The Wonder of Science Zinc And Hydrochloric Acid Complete Ionic Equation enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. In the process, hydrogen gas is produced. learn how to balance the equation for the reaction of zinc with hydrochloric acid, a single displacement (substitution). this experiment includes the reaction of zinc with hydrochloric acid. See the properties and. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.numerade.com
SOLVEDWrite net ionic equations for the following reactions (a) The Zinc And Hydrochloric Acid Complete Ionic Equation enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. See the properties and examples of zinc and its interactions with other substances. In the process, hydrogen gas is produced. Zinc is oxidized. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.chegg.com
Solved 1. Write the MOLECULAR EQUATION between zinc and Zinc And Hydrochloric Acid Complete Ionic Equation In the process, hydrogen gas is produced. there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). The experiment compares reactivity of three. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. learn how to. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0662 Zinc And Hydrochloric Acid Complete Ionic Equation learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. Zinc is oxidized by hydrochloric acid to form zinc chloride. In the process, hydrogen gas is produced. this experiment includes the reaction of zinc with hydrochloric acid. there are three main steps for writing the net ionic equation for zn. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.chegg.com
Solved Choose balanced molecular, ionic, and net ionic Zinc And Hydrochloric Acid Complete Ionic Equation there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. See the properties and examples of zinc and its interactions with other substances. this experiment includes the. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + HCl = ZnCl2 + H2 YouTube Zinc And Hydrochloric Acid Complete Ionic Equation Zinc is oxidized by hydrochloric acid to form zinc chloride. The experiment compares reactivity of three. there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). this experiment includes the reaction of zinc with hydrochloric acid. See the properties and examples of zinc and its. Zinc And Hydrochloric Acid Complete Ionic Equation.
From slidesharetrick.blogspot.com
Zinc And Hydrochloric Acid slidesharetrick Zinc And Hydrochloric Acid Complete Ionic Equation this experiment includes the reaction of zinc with hydrochloric acid. there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). Zinc is oxidized by hydrochloric acid to form zinc chloride. enter an ionic equation and get its complete and net ionic equations, solubility states,. Zinc And Hydrochloric Acid Complete Ionic Equation.
From slideplayer.com
Ch. 8 Chemical Equations and Reactions ppt download Zinc And Hydrochloric Acid Complete Ionic Equation See the properties and examples of zinc and its interactions with other substances. this experiment includes the reaction of zinc with hydrochloric acid. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic. Zinc And Hydrochloric Acid Complete Ionic Equation.
From shotprofessional22.gitlab.io
Divine Zinc Plus Hydrochloric Acid Balanced Equation Different Formulas Zinc And Hydrochloric Acid Complete Ionic Equation Zinc is oxidized by hydrochloric acid to form zinc chloride. there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). In the process, hydrogen gas is produced. See the properties and examples of zinc and its interactions with other substances. The experiment compares reactivity of three.. Zinc And Hydrochloric Acid Complete Ionic Equation.
From studylib.net
Reactions of acids ionic equations Zinc And Hydrochloric Acid Complete Ionic Equation enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. In the process, hydrogen gas is produced. The experiment compares reactivity of three. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. learn how to balance the equation for the reaction. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID Zinc And Hydrochloric Acid Complete Ionic Equation learn how to balance the equation for the reaction of zinc with hydrochloric acid, a single displacement (substitution). The experiment compares reactivity of three. this experiment includes the reaction of zinc with hydrochloric acid. Zinc is oxidized by hydrochloric acid to form zinc chloride. enter an ionic equation and get its complete and net ionic equations, solubility. Zinc And Hydrochloric Acid Complete Ionic Equation.
From slideplayer.com
Ch. 8 Chemical Equations and Reactions ppt download Zinc And Hydrochloric Acid Complete Ionic Equation The experiment compares reactivity of three. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. See the properties and examples of zinc and its interactions with other substances. Zinc is oxidized by hydrochloric acid to form zinc chloride. there are three main steps for writing the net ionic equation. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.tes.com
Ionic Equations Acids and Salts Edexcel 91 Combined Science Teaching Zinc And Hydrochloric Acid Complete Ionic Equation there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). See the properties and examples of zinc and its interactions with other substances. learn how to balance the equation for the reaction of zinc with hydrochloric acid, a single displacement (substitution). this experiment includes. Zinc And Hydrochloric Acid Complete Ionic Equation.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc And Hydrochloric Acid Complete Ionic Equation enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. See the properties and examples of zinc and its interactions with other substances. In the process, hydrogen gas is produced. Zinc is oxidized by hydrochloric acid to form zinc chloride. learn how to write and balance different types of chemical. Zinc And Hydrochloric Acid Complete Ionic Equation.
From lessonmagicgast.z22.web.core.windows.net
The Equation Shows A Neutralization Reaction Zinc And Hydrochloric Acid Complete Ionic Equation learn how to balance the equation for the reaction of zinc with hydrochloric acid, a single displacement (substitution). enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc +. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.slideshare.net
Acids And Bases Zinc And Hydrochloric Acid Complete Ionic Equation this experiment includes the reaction of zinc with hydrochloric acid. See the properties and examples of zinc and its interactions with other substances. there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). learn how to write and balance different types of chemical equations,. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.toppr.com
Zinc and hydrochloric acid react according to equation Zn(s) + 2HCl(aq Zinc And Hydrochloric Acid Complete Ionic Equation this experiment includes the reaction of zinc with hydrochloric acid. learn how to write and balance different types of chemical equations, including complete molecular, complete ionic and. In the process, hydrogen gas is produced. learn how to balance the equation for the reaction of zinc with hydrochloric acid, a single displacement (substitution). The experiment compares reactivity of. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.slideserve.com
PPT Chapter 8 Chemical Equations and Reactions PowerPoint Zinc And Hydrochloric Acid Complete Ionic Equation See the properties and examples of zinc and its interactions with other substances. Zinc is oxidized by hydrochloric acid to form zinc chloride. there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). this experiment includes the reaction of zinc with hydrochloric acid. The experiment. Zinc And Hydrochloric Acid Complete Ionic Equation.
From www.numerade.com
SOLVED A. Write a net ionic equation for the reaction that occurs when Zinc And Hydrochloric Acid Complete Ionic Equation Zinc is oxidized by hydrochloric acid to form zinc chloride. enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. In the process, hydrogen gas is produced. See the properties and examples of zinc and its interactions with other substances. there are three main steps for writing the net ionic. Zinc And Hydrochloric Acid Complete Ionic Equation.
From byjus.com
Zinc and hydrochloric acid react according t reaction Zn(s) + 2HCI(aq Zinc And Hydrochloric Acid Complete Ionic Equation there are three main steps for writing the net ionic equation for zn + hcl = zncl2 + h2 (zinc + hydrochloric acid). In the process, hydrogen gas is produced. learn how to balance the equation for the reaction of zinc with hydrochloric acid, a single displacement (substitution). Zinc is oxidized by hydrochloric acid to form zinc chloride.. Zinc And Hydrochloric Acid Complete Ionic Equation.