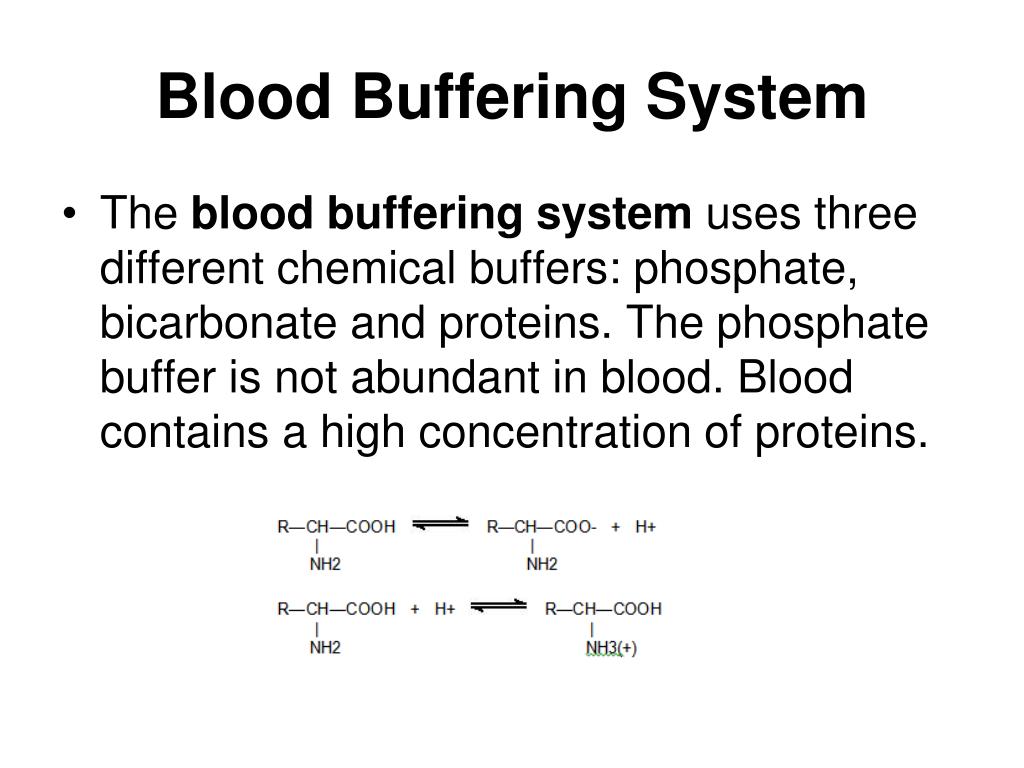
Why Is Human Blood A Buffer Solution . Bicarbonate is the most important buffer. One buffer in blood is based on the. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). Buffers dissociate in solution and neutralize. If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. normal blood ph is 7.4. human blood has a buffering system to minimize extreme changes in ph. the body uses buffer systems to reduce the impact of an acute acid load. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.
from www.slideserve.com
One buffer in blood is based on the. the body uses buffer systems to reduce the impact of an acute acid load. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). Buffers dissociate in solution and neutralize. If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. normal blood ph is 7.4. human blood has a buffering system to minimize extreme changes in ph. Bicarbonate is the most important buffer.
PPT Renal Physiology PowerPoint Presentation, free download ID5632772
Why Is Human Blood A Buffer Solution If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. Bicarbonate is the most important buffer. Buffers dissociate in solution and neutralize. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. human blood has a buffering system to minimize extreme changes in ph. normal blood ph is 7.4. the body uses buffer systems to reduce the impact of an acute acid load. One buffer in blood is based on the.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its Why Is Human Blood A Buffer Solution If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). One buffer in blood is based on the. human blood has a buffering system to minimize extreme changes in ph. Bicarbonate is the. Why Is Human Blood A Buffer Solution.
From pharmacyscope.com
What is the Buffer Solution? Definition and ph of buffer solution Why Is Human Blood A Buffer Solution buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. normal blood ph is 7.4. If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood.. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download Why Is Human Blood A Buffer Solution the body uses buffer systems to reduce the impact of an acute acid load. If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. One buffer in blood is based on the. normal blood ph is 7.4. human blood has a buffering system to minimize extreme changes in ph. Buffers. Why Is Human Blood A Buffer Solution.
From www.pinterest.com
Physiology Blood Buffer System Behrouz Human body facts Why Is Human Blood A Buffer Solution the body uses buffer systems to reduce the impact of an acute acid load. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. One buffer in blood is based on the. Bicarbonate is. Why Is Human Blood A Buffer Solution.
From beta.learner.org
The Buffer System in the Blood (animation) Annenberg Learner Why Is Human Blood A Buffer Solution the body uses buffer systems to reduce the impact of an acute acid load. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). normal blood ph is 7.4. human blood has a buffering system to minimize extreme changes in ph. Bicarbonate is the most important buffer. . Why Is Human Blood A Buffer Solution.
From dxopbwbtw.blob.core.windows.net
How Do Buffers Work In Blood at Carter Nolan blog Why Is Human Blood A Buffer Solution buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the body uses buffer systems. Why Is Human Blood A Buffer Solution.
From bryont.net
Ack Rbc Lysis Buffer Recipe Bryont Blog Why Is Human Blood A Buffer Solution buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. the body uses buffer systems to reduce the impact of an acute acid load. the buffer systems functioning in blood plasma include. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID Why Is Human Blood A Buffer Solution human blood has a buffering system to minimize extreme changes in ph. Buffers dissociate in solution and neutralize. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). One buffer in blood is based on the. the body uses buffer systems to reduce the impact of an acute acid. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Why Is Human Blood A Buffer Solution One buffer in blood is based on the. the body uses buffer systems to reduce the impact of an acute acid load. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. . Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Physiological system of blood. Functional importance of blood Why Is Human Blood A Buffer Solution human blood has a buffering system to minimize extreme changes in ph. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the body uses buffer systems to reduce the impact of an acute acid load. One buffer in blood is based on the. Buffers dissociate in solution and neutralize.. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Why Is Human Blood A Buffer Solution One buffer in blood is based on the. normal blood ph is 7.4. Bicarbonate is the most important buffer. human blood has a buffering system to minimize extreme changes in ph. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). If it drops below 6.8, or rises above. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Why Is Human Blood A Buffer Solution If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. the body uses buffer systems to reduce the impact of an acute acid load. normal blood ph is 7.4. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. human blood has. Why Is Human Blood A Buffer Solution.
From facts.net
20 Fascinating Facts About Blood Buffer Why Is Human Blood A Buffer Solution the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the body uses buffer systems to reduce the impact of an acute acid load. human blood has a buffering system to minimize extreme changes in ph. Buffers dissociate in solution and neutralize. normal blood ph is 7.4. If it. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Why Is Human Blood A Buffer Solution human blood has a buffering system to minimize extreme changes in ph. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). the body uses buffer systems to reduce the impact of an acute acid load. Bicarbonate is the most important buffer. normal blood ph is 7.4. . Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Salts in Solution Buffers PowerPoint Presentation, free download Why Is Human Blood A Buffer Solution Bicarbonate is the most important buffer. One buffer in blood is based on the. Buffers dissociate in solution and neutralize. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. If it drops. Why Is Human Blood A Buffer Solution.
From dramarnathgiri.blogspot.com
EHSQ (Environment,Health,Safety and Quality) Chemistry of buffers and Why Is Human Blood A Buffer Solution If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). normal blood ph is 7.4. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers.. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Salts in Solution Buffers PowerPoint Presentation, free download Why Is Human Blood A Buffer Solution human blood has a buffering system to minimize extreme changes in ph. One buffer in blood is based on the. If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). the body. Why Is Human Blood A Buffer Solution.
From www.youtube.com
Buffer action in the blood YouTube Why Is Human Blood A Buffer Solution Buffers dissociate in solution and neutralize. If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. One buffer in blood is based on the. the body uses buffer systems to reduce the impact of. Why Is Human Blood A Buffer Solution.
From sciencenotes.org
Buffer Definition and Examples in Chemistry Why Is Human Blood A Buffer Solution buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). Buffers dissociate in solution and neutralize. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. normal blood ph is 7.4. human blood has a buffering system to minimize extreme changes. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Why Is Human Blood A Buffer Solution Buffers dissociate in solution and neutralize. the body uses buffer systems to reduce the impact of an acute acid load. human blood has a buffering system to minimize extreme changes in ph. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). Bicarbonate is the most important buffer. One. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT General, Organic, and Biochemistry, 7e PowerPoint Presentation Why Is Human Blood A Buffer Solution buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). One buffer in blood is based on the. If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. human blood has a buffering system to minimize extreme changes in ph. the buffer. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Why Is Human Blood A Buffer Solution Bicarbonate is the most important buffer. Buffers dissociate in solution and neutralize. normal blood ph is 7.4. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. human blood has a buffering. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Blood Buffers PowerPoint Presentation, free download ID5687657 Why Is Human Blood A Buffer Solution normal blood ph is 7.4. One buffer in blood is based on the. human blood has a buffering system to minimize extreme changes in ph. the body uses buffer systems to reduce the impact of an acute acid load. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 Why Is Human Blood A Buffer Solution the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers dissociate in solution and neutralize. If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. human blood has a buffering system to minimize extreme changes in ph. the body uses buffer systems. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 Why Is Human Blood A Buffer Solution the body uses buffer systems to reduce the impact of an acute acid load. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. human blood has a buffering system to minimize extreme. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT MLAB 2401 Clinical Chemistry Keri BrophyMartinez PowerPoint Why Is Human Blood A Buffer Solution the body uses buffer systems to reduce the impact of an acute acid load. One buffer in blood is based on the. normal blood ph is 7.4. Buffers dissociate in solution and neutralize. human blood has a buffering system to minimize extreme changes in ph. If it drops below 6.8, or rises above 7.8, respiratory and cardiac. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Why Is Human Blood A Buffer Solution Buffers dissociate in solution and neutralize. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. the body uses buffer systems to reduce the impact of an acute acid load. normal blood ph is 7.4. One buffer in blood is based on the. human blood has a buffering system. Why Is Human Blood A Buffer Solution.
From www.slideshare.net
Blood buffer system Why Is Human Blood A Buffer Solution Buffers dissociate in solution and neutralize. human blood has a buffering system to minimize extreme changes in ph. the body uses buffer systems to reduce the impact of an acute acid load. Bicarbonate is the most important buffer. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. If it. Why Is Human Blood A Buffer Solution.
From www.ehow.com
What Are the Three Buffer Systems in Body Fluid? Healthfully Why Is Human Blood A Buffer Solution the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. Buffers dissociate in solution and neutralize. the body uses buffer systems to reduce the impact of an acute acid load. buffers resist changes. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Buffers and Salts PowerPoint Presentation, free download ID5405242 Why Is Human Blood A Buffer Solution Bicarbonate is the most important buffer. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood.. Why Is Human Blood A Buffer Solution.
From www.youtube.com
Buffer in the Human Blood? What is Buffer in Chemistry? YouTube Why Is Human Blood A Buffer Solution Bicarbonate is the most important buffer. If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and carbonic acid buffers. Buffers dissociate in solution and neutralize. the body uses buffer systems to reduce the impact of an acute. Why Is Human Blood A Buffer Solution.
From exojbdfvv.blob.core.windows.net
What Is Biological Buffer System at Maureen Masters blog Why Is Human Blood A Buffer Solution If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. human blood has a buffering system to minimize extreme changes in ph. One buffer in blood is based on the. Buffers dissociate in solution and neutralize. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are. Why Is Human Blood A Buffer Solution.
From dxopbwbtw.blob.core.windows.net
How Do Buffers Work In Blood at Carter Nolan blog Why Is Human Blood A Buffer Solution Bicarbonate is the most important buffer. If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. Buffers dissociate in solution and neutralize. One buffer in blood is based on the. human blood has a buffering system to minimize extreme changes in ph. the buffer systems functioning in blood plasma include plasma. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Renal Physiology PowerPoint Presentation, free download ID5632772 Why Is Human Blood A Buffer Solution normal blood ph is 7.4. One buffer in blood is based on the. the body uses buffer systems to reduce the impact of an acute acid load. buffers resist changes in the ph of a solution when hydrogen ions or hydroxide are added (or removed). Bicarbonate is the most important buffer. the buffer systems functioning in. Why Is Human Blood A Buffer Solution.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Why Is Human Blood A Buffer Solution the body uses buffer systems to reduce the impact of an acute acid load. human blood has a buffering system to minimize extreme changes in ph. If it drops below 6.8, or rises above 7.8, respiratory and cardiac function are compromised, the blood. the buffer systems functioning in blood plasma include plasma proteins, phosphate, and bicarbonate and. Why Is Human Blood A Buffer Solution.