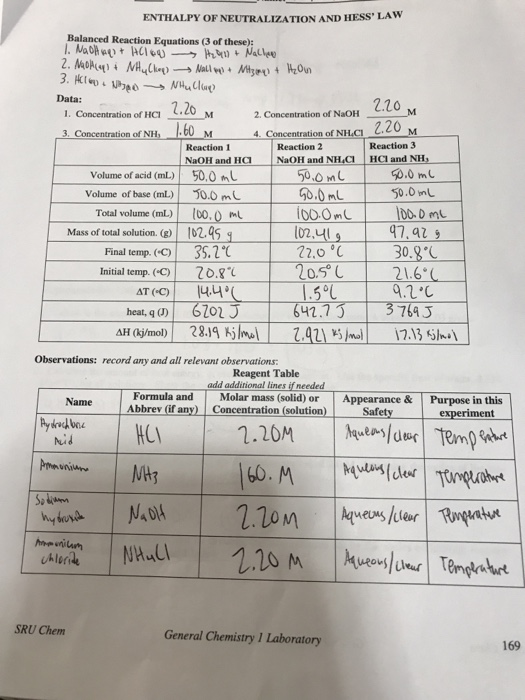
Calorimetry Experiment Neutralization . The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an. Pag 3.1 determination of the enthalpy change of neutralisation. Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. The experimental apparatus consists of a. This is an iot lab, where you will be asked to design the experiment and your ta will perform it while streaming data in real time to a google sheet. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. The enthalpy change of acid base neutralization reactions will be determined. We used a constructed calorimeter and. The principle of these calorimetry experiments is to mix stoichiometric quantities of acid and alkali then measure the temperature change over the course of a few minutes; The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown above This experiment introduces the technique of calorimetry.
from www.chegg.com
Pag 3.1 determination of the enthalpy change of neutralisation. We used a constructed calorimeter and. Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. This is an iot lab, where you will be asked to design the experiment and your ta will perform it while streaming data in real time to a google sheet. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. The principle of these calorimetry experiments is to mix stoichiometric quantities of acid and alkali then measure the temperature change over the course of a few minutes; The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown above The enthalpy change of acid base neutralization reactions will be determined. This experiment introduces the technique of calorimetry. The experimental apparatus consists of a.
Solved ENTHALPY OF NEUTRALIZATION AND HESS LAW Post
Calorimetry Experiment Neutralization The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown above The principle of these calorimetry experiments is to mix stoichiometric quantities of acid and alkali then measure the temperature change over the course of a few minutes; The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown above The experimental apparatus consists of a. This experiment introduces the technique of calorimetry. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. This is an iot lab, where you will be asked to design the experiment and your ta will perform it while streaming data in real time to a google sheet. The enthalpy change of acid base neutralization reactions will be determined. Pag 3.1 determination of the enthalpy change of neutralisation. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an. We used a constructed calorimeter and. Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation.
From www.youtube.com
Heat of Reaction from a Calorimeter (Example) YouTube Calorimetry Experiment Neutralization The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by. Calorimetry Experiment Neutralization.
From www.youtube.com
CALORIMETRY EXAMPLE 3 YouTube Calorimetry Experiment Neutralization This is an iot lab, where you will be asked to design the experiment and your ta will perform it while streaming data in real time to a google sheet. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). The goal of this experiment is to. Calorimetry Experiment Neutralization.
From www.tessshebaylo.com
Equation For Determining Calorimetry Tessshebaylo Calorimetry Experiment Neutralization Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. The principle of these calorimetry experiments is to mix stoichiometric quantities of acid and alkali then measure the temperature change over the course of a few minutes; This is an iot lab, where you will be asked to design. Calorimetry Experiment Neutralization.
From www.chegg.com
Solved Experimental Chemistry I Part II Heat of Calorimetry Experiment Neutralization We used a constructed calorimeter and. This is an iot lab, where you will be asked to design the experiment and your ta will perform it while streaming data in real time to a google sheet. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Introduction to the. Calorimetry Experiment Neutralization.
From www.chegg.com
Solved EXPERIMENT REPORT SHEET Heat of Neutralization A. Calorimetry Experiment Neutralization We used a constructed calorimeter and. This experiment introduces the technique of calorimetry. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. This is an iot lab, where you will be asked to design the experiment and your ta will perform it while streaming data in real time. Calorimetry Experiment Neutralization.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimetry Experiment Neutralization Pag 3.1 determination of the enthalpy change of neutralisation. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an. We used a constructed calorimeter and. Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat. Calorimetry Experiment Neutralization.
From www.nagwa.com
Question Video Determining the Suitability of Experimental Apparatus Calorimetry Experiment Neutralization Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an. We used a constructed calorimeter and. The principle of these calorimetry experiments is to mix stoichiometric quantities of acid and alkali then measure the temperature change over the course of a few minutes;. Calorimetry Experiment Neutralization.
From www.chegg.com
Solved EXPERIMENT 11 POST LABORATORY Heat of AcidBase Calorimetry Experiment Neutralization The experimental apparatus consists of a. The principle of these calorimetry experiments is to mix stoichiometric quantities of acid and alkali then measure the temperature change over the course of a few minutes; Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an.. Calorimetry Experiment Neutralization.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Calorimetry Experiment Neutralization Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. The principle of these calorimetry experiments is to mix stoichiometric quantities of acid and alkali then measure the temperature change over the course of a few minutes; The heat of neutralization that is lost in the chemical. Calorimetry Experiment Neutralization.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记4.1.4 Determining Enthalpy Change of Calorimetry Experiment Neutralization This is an iot lab, where you will be asked to design the experiment and your ta will perform it while streaming data in real time to a google sheet. The experimental apparatus consists of a. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. Abstract the purpose of this experiment was to determine. Calorimetry Experiment Neutralization.
From www.chegg.com
Solved ENTHALPY OF NEUTRALIZATION AND HESS LAW Post Calorimetry Experiment Neutralization The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. We used a constructed calorimeter and. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown above Pag 3.1 determination of the enthalpy change of neutralisation. Introduction to the technique of calorimetry, in which the heat evolved (given off). Calorimetry Experiment Neutralization.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimetry Experiment Neutralization Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. This is an iot lab, where you will be asked to design the experiment and your ta will perform it while streaming data in real time to a google sheet. The heat of neutralization that is lost. Calorimetry Experiment Neutralization.
From jimoxobaxib.balmettes.com
Heat Of Neutralization Calorimetry Experiment Neutralization The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). We used a constructed calorimeter and. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an. Simple calorimetry experiments can. Calorimetry Experiment Neutralization.
From www.studocu.com
Calorimetry LAB Report Calorimetry Enthalpy of Neutralization Lab Calorimetry Experiment Neutralization The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. The enthalpy change of acid base neutralization reactions will be determined. The apparatus needed. Calorimetry Experiment Neutralization.
From www.chegg.com
Solved REPORT SHEET EXPERIMENT Heat Of Neutralization 28 Calorimetry Experiment Neutralization The enthalpy change of acid base neutralization reactions will be determined. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown above This experiment introduces the technique of calorimetry. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes. Calorimetry Experiment Neutralization.
From online-learning-college.com
Calorimetry experiments Displacement reactions Neutralisation Calorimetry Experiment Neutralization The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown above The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). The principle of these calorimetry experiments is to mix stoichiometric quantities of acid and alkali then measure the temperature change. Calorimetry Experiment Neutralization.
From socratic.org
A student conducting a calorimetry investigation determines a negative Calorimetry Experiment Neutralization This experiment introduces the technique of calorimetry. Pag 3.1 determination of the enthalpy change of neutralisation. We used a constructed calorimeter and. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is. Calorimetry Experiment Neutralization.
From www.youtube.com
Heat of Reaction (Calorimetry) Experiment YouTube Calorimetry Experiment Neutralization Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. The enthalpy change of acid base neutralization reactions will be determined. The heat of neutralization that. Calorimetry Experiment Neutralization.
From www.academia.edu
(DOC) Enthalpy of Neutralization Procedure/Experiment Jonniel Vince Calorimetry Experiment Neutralization The enthalpy change of acid base neutralization reactions will be determined. The principle of these calorimetry experiments is to mix stoichiometric quantities of acid and alkali then measure the temperature change over the course of a few minutes; This is an iot lab, where you will be asked to design the experiment and your ta will perform it while streaming. Calorimetry Experiment Neutralization.
From www.abhayjere.com
Calorimetry Worksheet Answer Key Calorimetry Experiment Neutralization This experiment introduces the technique of calorimetry. The experimental apparatus consists of a. The principle of these calorimetry experiments is to mix stoichiometric quantities of acid and alkali then measure the temperature change over the course of a few minutes; The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. We used a constructed calorimeter. Calorimetry Experiment Neutralization.
From www.youtube.com
General Chemistry II Solving Calorimetry Problems Neutralization Calorimetry Experiment Neutralization Abstract the purpose of this experiment was to determine the heat capacity of the calorimeter and the heat of neutralization of different reactions. The principle of these calorimetry experiments is to mix stoichiometric quantities of acid and alkali then measure the temperature change over the course of a few minutes; This is an iot lab, where you will be asked. Calorimetry Experiment Neutralization.
From www.studocu.com
Calorimetry anf heat of neutralization exp BSc V sem Revised 11/2015 Calorimetry Experiment Neutralization The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown above This is an iot lab, where you will be asked to design the experiment and your ta will perform it while streaming data in real time to a. Calorimetry Experiment Neutralization.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.2 Describe Simple Calorimetry Experiments for Calorimetry Experiment Neutralization The principle of these calorimetry experiments is to mix stoichiometric quantities of acid and alkali then measure the temperature change over the course of a few minutes; Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. This experiment introduces the technique of calorimetry. The heat of neutralization that. Calorimetry Experiment Neutralization.
From www.researchgate.net
(PDF) ChemDuinoCalorimetry to Determine the Enthalpy Change of Calorimetry Experiment Neutralization Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an. The enthalpy change of acid base neutralization reactions will be determined. This is an iot lab, where you will be asked to design the experiment and your ta will perform it while streaming. Calorimetry Experiment Neutralization.
From klarxnzah.blob.core.windows.net
Calorimetry Experiment With Different Metals at David Lytton blog Calorimetry Experiment Neutralization The enthalpy change of acid base neutralization reactions will be determined. We used a constructed calorimeter and. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an. This experiment introduces the technique of calorimetry. The principle of these calorimetry experiments is to mix. Calorimetry Experiment Neutralization.
From slideplayer.com
Enthalpy Starter BACK OF BOOK ppt download Calorimetry Experiment Neutralization The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). The apparatus needed to carry out an enthalpy of reaction in solution calorimetry experiment is shown above The principle of these calorimetry experiments is to mix stoichiometric quantities of acid and alkali then measure the temperature change. Calorimetry Experiment Neutralization.
From www.chegg.com
Solved Calorimetry Experiment Neutralization The principle of these calorimetry experiments is to mix stoichiometric quantities of acid and alkali then measure the temperature change over the course of a few minutes; The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. We used a constructed calorimeter and. Simple calorimetry experiments can be used to calculate the heat energy transferred. Calorimetry Experiment Neutralization.
From allanfersmaxwell.blogspot.com
How to Calculate Delta H of Neutralization Calorimetry Experiment Neutralization Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. The enthalpy change of acid base neutralization reactions will be determined. Abstract the purpose of this experiment was. Calorimetry Experiment Neutralization.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Calorimetry Experiment Neutralization The enthalpy change of acid base neutralization reactions will be determined. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an. The goal of this experiment is to determine the molar enthalpy of neutralization using calorimetry. The heat of neutralization that is lost. Calorimetry Experiment Neutralization.
From www.chegg.com
Solved (2pts) Constant Pressure Calorimetry Are you Calorimetry Experiment Neutralization We used a constructed calorimeter and. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. The enthalpy change of acid base neutralization reactions will be determined. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by. Calorimetry Experiment Neutralization.
From www.studocu.com
P calorimetry 25 lab report StuDocu Calorimetry Experiment Neutralization We used a constructed calorimeter and. The experimental apparatus consists of a. Pag 3.1 determination of the enthalpy change of neutralisation. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). The enthalpy change of acid base neutralization reactions will be determined. The principle of these calorimetry. Calorimetry Experiment Neutralization.
From studylib.net
Calorimetry Heat of Neutralisation Calorimetry Experiment Neutralization This experiment introduces the technique of calorimetry. Pag 3.1 determination of the enthalpy change of neutralisation. This is an iot lab, where you will be asked to design the experiment and your ta will perform it while streaming data in real time to a google sheet. Abstract the purpose of this experiment was to determine the heat capacity of the. Calorimetry Experiment Neutralization.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Experiment Neutralization This is an iot lab, where you will be asked to design the experiment and your ta will perform it while streaming data in real time to a google sheet. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an. Pag 3.1 determination. Calorimetry Experiment Neutralization.
From www.chegg.com
Solved CALORIMETRY ENTHALPY OF NEUTRALIZATION LABORATORY Calorimetry Experiment Neutralization We used a constructed calorimeter and. The heat of neutralization that is lost in the chemical reaction (the system) is gained by the calorimeter and its contents (the surroundings). Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a chemical reaction is inferred by measuring temperature changes in an. The experimental apparatus consists. Calorimetry Experiment Neutralization.
From dxoazusse.blob.core.windows.net
Food Calorimetry Equation at Jesse Freeman blog Calorimetry Experiment Neutralization The principle of these calorimetry experiments is to mix stoichiometric quantities of acid and alkali then measure the temperature change over the course of a few minutes; This experiment introduces the technique of calorimetry. Pag 3.1 determination of the enthalpy change of neutralisation. Introduction to the technique of calorimetry, in which the heat evolved (given off) or absorbed by a. Calorimetry Experiment Neutralization.