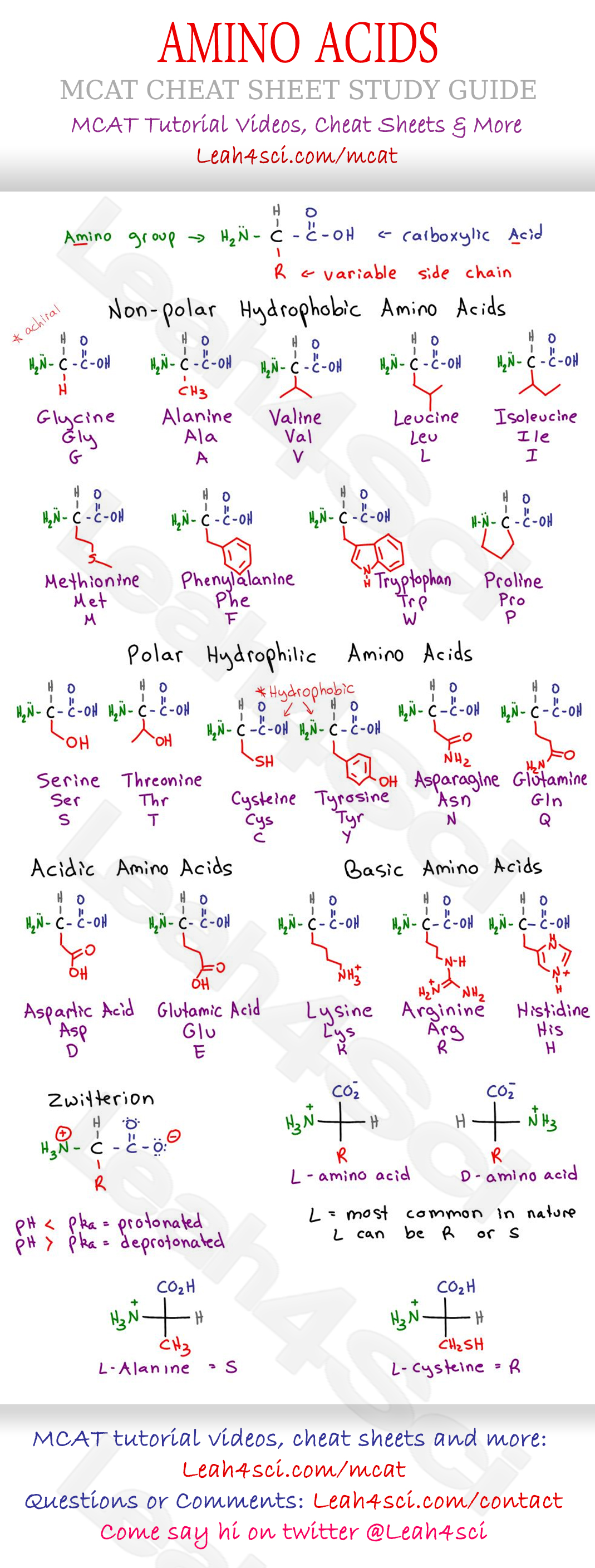
L Vs D Amino Acids Mcat . The question for this was: With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. Can someone please explain why choice c is l and why choice d would not be l? As we’ll discuss, amino acids can be described in terms of their absolute configuration (r and s notation) or their relative configuration (l and d notation). This is based upon the presence of an. The chirality of biological molecules becomes quite important, as only l. The l designation of the amino acids used in peptide synthesis is based on the. L and d configurations of amino acids are different from r and s configurations of organic molecules. There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids.
from leah4sci.com
With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. The l designation of the amino acids used in peptide synthesis is based on the. This is based upon the presence of an. Can someone please explain why choice c is l and why choice d would not be l? As we’ll discuss, amino acids can be described in terms of their absolute configuration (r and s notation) or their relative configuration (l and d notation). There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. L and d configurations of amino acids are different from r and s configurations of organic molecules. The question for this was: The chirality of biological molecules becomes quite important, as only l.
Amino Acid Chart MCAT Cheat Sheet Study Guide
L Vs D Amino Acids Mcat L and d configurations of amino acids are different from r and s configurations of organic molecules. Can someone please explain why choice c is l and why choice d would not be l? As we’ll discuss, amino acids can be described in terms of their absolute configuration (r and s notation) or their relative configuration (l and d notation). With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. The question for this was: The l designation of the amino acids used in peptide synthesis is based on the. The chirality of biological molecules becomes quite important, as only l. This is based upon the presence of an. L and d configurations of amino acids are different from r and s configurations of organic molecules.
From www.cannabismagazine.net
Los aminoácidos en el cultivo de cannabis (II) L Vs D Amino Acids Mcat The l designation of the amino acids used in peptide synthesis is based on the. This is based upon the presence of an. L and d configurations of amino acids are different from r and s configurations of organic molecules. With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. The question for this. L Vs D Amino Acids Mcat.
From www.etsy.com
MCAT Amino Acids PDF Etsy L Vs D Amino Acids Mcat As we’ll discuss, amino acids can be described in terms of their absolute configuration (r and s notation) or their relative configuration (l and d notation). With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. This is based upon the presence of an. Can someone please explain why choice c is l and. L Vs D Amino Acids Mcat.
From leah4sci.com
Amino Acid Chart MCAT Cheat Sheet Study Guide L Vs D Amino Acids Mcat L and d configurations of amino acids are different from r and s configurations of organic molecules. The question for this was: As we’ll discuss, amino acids can be described in terms of their absolute configuration (r and s notation) or their relative configuration (l and d notation). Can someone please explain why choice c is l and why choice. L Vs D Amino Acids Mcat.
From bio.libretexts.org
12.1.5 Proteins Biology LibreTexts L Vs D Amino Acids Mcat This is based upon the presence of an. L and d configurations of amino acids are different from r and s configurations of organic molecules. The chirality of biological molecules becomes quite important, as only l. The question for this was: As we’ll discuss, amino acids can be described in terms of their absolute configuration (r and s notation) or. L Vs D Amino Acids Mcat.
From aminoco.com
L vs. DAmino Acids The Amino Company L Vs D Amino Acids Mcat Can someone please explain why choice c is l and why choice d would not be l? With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. The chirality of biological molecules becomes quite important, as only l. L and d configurations of amino acids are different from r and s configurations of organic. L Vs D Amino Acids Mcat.
From www.pinterest.com.mx
Pin on MCAT Bio / Biochemistry Tutorials and Resources L Vs D Amino Acids Mcat L and d configurations of amino acids are different from r and s configurations of organic molecules. There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. This is based upon. L Vs D Amino Acids Mcat.
From cwsimons.com
Structure of Amino Acids and Proteins L Vs D Amino Acids Mcat There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. This is based upon the presence of an. The l designation of the amino acids used in peptide synthesis is based. L Vs D Amino Acids Mcat.
From leah4sci.com
Amino Acid Charge in Zwitterions and Isoelectric Point MCAT Tutorial L Vs D Amino Acids Mcat Can someone please explain why choice c is l and why choice d would not be l? The l designation of the amino acids used in peptide synthesis is based on the. As we’ll discuss, amino acids can be described in terms of their absolute configuration (r and s notation) or their relative configuration (l and d notation). L and. L Vs D Amino Acids Mcat.
From jackwestin.com
MCAT Amino Acids Cheat Sheet L Vs D Amino Acids Mcat With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. This is based upon the presence of an. Can someone please explain why choice c is l and why choice d. L Vs D Amino Acids Mcat.
From klamttbcu.blob.core.windows.net
Amino Acid Letter I at Dawn Heath blog L Vs D Amino Acids Mcat L and d configurations of amino acids are different from r and s configurations of organic molecules. There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. The l designation of the amino acids used in peptide synthesis is based on the. The chirality of biological molecules. L Vs D Amino Acids Mcat.
From etna.com.pe
Art & Collectibles Amino Acids Tested on the MCAT Poster *DIGITAL L Vs D Amino Acids Mcat The chirality of biological molecules becomes quite important, as only l. This is based upon the presence of an. As we’ll discuss, amino acids can be described in terms of their absolute configuration (r and s notation) or their relative configuration (l and d notation). With the exception of glycine, each of the amino acids incorporated into proteins displays optical. L Vs D Amino Acids Mcat.
From quizlet.com
MCAT Amino Acids Diagram Quizlet L Vs D Amino Acids Mcat With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. The chirality of biological molecules becomes quite important, as only l. The l designation of the amino acids used in peptide synthesis is based on the. Can someone please explain why choice c is l and why choice d would not be l? This. L Vs D Amino Acids Mcat.
From www.medschoolcoach.com
Amino Acid Configuration and Structure MCAT Biochemistry MedSchoolCoach L Vs D Amino Acids Mcat Can someone please explain why choice c is l and why choice d would not be l? The question for this was: There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. As we’ll discuss, amino acids can be described in terms of their absolute configuration (r. L Vs D Amino Acids Mcat.
From onlinesciencenotes.com
Amino acids General properties and classification Online Science Notes L Vs D Amino Acids Mcat The chirality of biological molecules becomes quite important, as only l. This is based upon the presence of an. With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. As we’ll discuss, amino acids can be described in terms of their absolute configuration (r and s notation) or their relative configuration (l and d. L Vs D Amino Acids Mcat.
From www.pinterest.de
Pin på med L Vs D Amino Acids Mcat The question for this was: This is based upon the presence of an. The chirality of biological molecules becomes quite important, as only l. L and d configurations of amino acids are different from r and s configurations of organic molecules. There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the. L Vs D Amino Acids Mcat.
From www.compoundchem.com
A Brief Guide to the Twenty Common Amino Acids Compound Interest L Vs D Amino Acids Mcat There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. L and d configurations of amino acids are different from r and s configurations of organic molecules. The chirality of biological molecules becomes quite important, as only l. The question for this was: This is based upon. L Vs D Amino Acids Mcat.
From www.scienceabc.com
What Are The Two Rare Amino Acids? » ScienceABC L Vs D Amino Acids Mcat There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. As we’ll discuss, amino acids can be described in terms of their absolute configuration (r and s notation) or their relative configuration (l and d notation). The chirality of biological molecules becomes quite important, as only l.. L Vs D Amino Acids Mcat.
From kin.naver.com
아미노산 관련 질문 지식iN L Vs D Amino Acids Mcat L and d configurations of amino acids are different from r and s configurations of organic molecules. Can someone please explain why choice c is l and why choice d would not be l? The l designation of the amino acids used in peptide synthesis is based on the. This is based upon the presence of an. As we’ll discuss,. L Vs D Amino Acids Mcat.
From www.scienceabc.com
What Are The Two Rare Amino Acids? » ScienceABC L Vs D Amino Acids Mcat The question for this was: L and d configurations of amino acids are different from r and s configurations of organic molecules. The chirality of biological molecules becomes quite important, as only l. The l designation of the amino acids used in peptide synthesis is based on the. There are d and l enantiomers, structurally identical molecules with opposed absolute. L Vs D Amino Acids Mcat.
From www.pinterest.co.uk
Amino Acids Chemistry, Biochemistry & Nutrition Biochemistry L Vs D Amino Acids Mcat The chirality of biological molecules becomes quite important, as only l. As we’ll discuss, amino acids can be described in terms of their absolute configuration (r and s notation) or their relative configuration (l and d notation). The l designation of the amino acids used in peptide synthesis is based on the. There are d and l enantiomers, structurally identical. L Vs D Amino Acids Mcat.
From www.etsy.com
MCAT Amino Acids PDF Etsy L Vs D Amino Acids Mcat This is based upon the presence of an. There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. The chirality of biological molecules becomes quite important, as only l. With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. L and. L Vs D Amino Acids Mcat.
From www.medschoolcoach.com
Amino Acid Classification MCAT Biochemistry MedSchoolCoach L Vs D Amino Acids Mcat This is based upon the presence of an. Can someone please explain why choice c is l and why choice d would not be l? The l designation of the amino acids used in peptide synthesis is based on the. As we’ll discuss, amino acids can be described in terms of their absolute configuration (r and s notation) or their. L Vs D Amino Acids Mcat.
From chemistry.com.pk
A Brief Introduction of Amino Acids The Building Blocks of Proteins L Vs D Amino Acids Mcat With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. The chirality of biological molecules becomes quite important, as only l. There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. The question for this was: L and d configurations of. L Vs D Amino Acids Mcat.
From klamjlrxw.blob.core.windows.net
Amino Acids And Their Abbreviations at Cornelius Venegas blog L Vs D Amino Acids Mcat The chirality of biological molecules becomes quite important, as only l. L and d configurations of amino acids are different from r and s configurations of organic molecules. With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. Can someone please explain why choice c is l and why choice d would not be. L Vs D Amino Acids Mcat.
From exozbdaug.blob.core.windows.net
Difference Between L Amino Acid And D Amino Acid at Kathy Newman blog L Vs D Amino Acids Mcat This is based upon the presence of an. There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. The chirality of biological molecules becomes quite important, as only l. The question for this was: With the exception of glycine, each of the amino acids incorporated into proteins. L Vs D Amino Acids Mcat.
From kierra-has-combs.blogspot.com
Amino Acids Are Monomers of Which of the Following Biochemicals L Vs D Amino Acids Mcat With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. Can someone please explain why choice c is l and why choice d would not be l? L and d configurations of amino acids are different from r and s configurations of organic molecules. As we’ll discuss, amino acids can be described in terms. L Vs D Amino Acids Mcat.
From brilliant.org
Chemistry Of Nutrition Brilliant Math & Science Wiki L Vs D Amino Acids Mcat The question for this was: As we’ll discuss, amino acids can be described in terms of their absolute configuration (r and s notation) or their relative configuration (l and d notation). Can someone please explain why choice c is l and why choice d would not be l? The chirality of biological molecules becomes quite important, as only l. There. L Vs D Amino Acids Mcat.
From pediaa.com
What is the Difference Between L and D Amino Acids L Vs D Amino Acids Mcat The question for this was: With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. The l designation of the amino acids used in peptide synthesis is based on the. There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. The. L Vs D Amino Acids Mcat.
From bio.libretexts.org
2.5.4 Amino Acids Biology LibreTexts L Vs D Amino Acids Mcat The l designation of the amino acids used in peptide synthesis is based on the. The question for this was: There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. L and d configurations of amino acids are different from r and s configurations of organic molecules.. L Vs D Amino Acids Mcat.
From www.medschoolcoach.com
Amino Acid Classification MCAT Biochemistry MedSchoolCoach L Vs D Amino Acids Mcat Can someone please explain why choice c is l and why choice d would not be l? This is based upon the presence of an. There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. The chirality of biological molecules becomes quite important, as only l. L. L Vs D Amino Acids Mcat.
From exozzbprf.blob.core.windows.net
Basic Amino Acids Have A + Charge at Thomas Auld blog L Vs D Amino Acids Mcat There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. Can someone please explain why choice c is l and why choice d would not be l? With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. As we’ll discuss, amino. L Vs D Amino Acids Mcat.
From joisuytfc.blob.core.windows.net
Amino Acid Residue Letters at Elsie Winkelman blog L Vs D Amino Acids Mcat The question for this was: There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. This is based upon the presence of an. The l designation of the amino acids used. L Vs D Amino Acids Mcat.
From joiawekwj.blob.core.windows.net
Amino Acids With NonIonizable Side Chains Are Zwitterions When They L Vs D Amino Acids Mcat With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. L and d configurations of amino acids are different from r and s configurations of organic molecules. There are d and l enantiomers, structurally identical molecules with opposed absolute configurations around the chiral carbon, for the 19 chiral amino acids. This is based upon. L Vs D Amino Acids Mcat.
From loehbpcor.blob.core.windows.net
L Vs D Configuration Amino Acids at Twila Messing blog L Vs D Amino Acids Mcat The chirality of biological molecules becomes quite important, as only l. With the exception of glycine, each of the amino acids incorporated into proteins displays optical activity. This is based upon the presence of an. As we’ll discuss, amino acids can be described in terms of their absolute configuration (r and s notation) or their relative configuration (l and d. L Vs D Amino Acids Mcat.