Error In Calorimetry Experiment . Learn how to perform and analyse the calorimetry practical and ace your next chemistry practical assessment. have a chemistry practical on the calorimetry experiment? Calorimetry is used to measure. The heat of the reaction cannot be. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. If δh is positive, the reaction is endothermic. if δh is negative, the reaction is exothermic. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. the calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. Chemical reactions involve the release or consumption of energy, usually. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. It is impossible to obtain an exact measurement due to the lack of precision of instruments and the.
from www.numerade.com
It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. if δh is negative, the reaction is exothermic. have a chemistry practical on the calorimetry experiment? Calorimetry is used to measure. The heat of the reaction cannot be. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. the calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. If δh is positive, the reaction is endothermic. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. Learn how to perform and analyse the calorimetry practical and ace your next chemistry practical assessment.
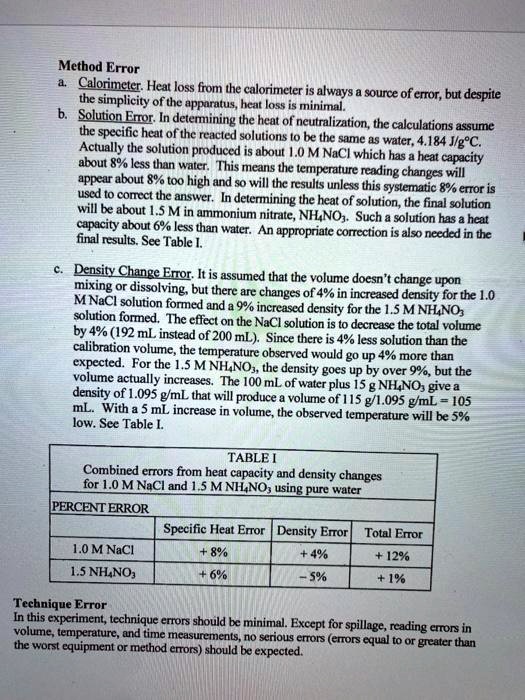
SOLVED Method Error Calorimeter Heat loss from the calorimeter is
Error In Calorimetry Experiment Calorimetry is used to measure. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. If δh is positive, the reaction is endothermic. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. the calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. Calorimetry is used to measure. Learn how to perform and analyse the calorimetry practical and ace your next chemistry practical assessment. The heat of the reaction cannot be. if δh is negative, the reaction is exothermic. Chemical reactions involve the release or consumption of energy, usually. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. have a chemistry practical on the calorimetry experiment?
From www.slideserve.com
PPT Chapter 14 Heat PowerPoint Presentation, free download ID6832402 Error In Calorimetry Experiment Chemical reactions involve the release or consumption of energy, usually. It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. Calorimetry is used to measure. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Learn how to perform. Error In Calorimetry Experiment.
From www.scribd.com
Experiment 1 Bomb Calorimetry Calorimetry Heat Error In Calorimetry Experiment the calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. If δh is positive, the reaction is endothermic. It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. The heat of the reaction cannot be. Learn how to perform and. Error In Calorimetry Experiment.
From www.numerade.com
SOLVED Explain how using large quantities in calorimetry leads to Error In Calorimetry Experiment Learn how to perform and analyse the calorimetry practical and ace your next chemistry practical assessment. if δh is negative, the reaction is exothermic. If δh is positive, the reaction is endothermic. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. the calorimeter constant. Error In Calorimetry Experiment.
From erwinnuza.blogspot.com
Types Of Experimental Errors Reasons for Error in a Chemistry Error In Calorimetry Experiment if δh is negative, the reaction is exothermic. It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. Chemical reactions involve the release or consumption of energy, usually. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. the calorimeter. Error In Calorimetry Experiment.
From sites.google.com
3.2.1 (e) Q=mcΔT Ellesmere OCR A level Chemistry Error In Calorimetry Experiment revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. Learn how to perform and analyse the calorimetry practical and ace your next chemistry practical assessment. It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. in this experiment you will. Error In Calorimetry Experiment.
From www.researchgate.net
Experimental procedure and raw results of calorimetry experiments. a Error In Calorimetry Experiment the calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. Chemical reactions involve the release or consumption of energy, usually. The heat of the reaction cannot be. one technique we can use to measure the amount of heat involved in a chemical or physical process is known. Error In Calorimetry Experiment.
From www.youtube.com
Heat of Reaction (Calorimetry) Experiment YouTube Error In Calorimetry Experiment If δh is positive, the reaction is endothermic. Calorimetry is used to measure. have a chemistry practical on the calorimetry experiment? one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Chemical reactions involve the release or consumption of energy, usually. the calorimeter constant ccal. Error In Calorimetry Experiment.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Error In Calorimetry Experiment have a chemistry practical on the calorimetry experiment? the calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. Calorimetry is used to measure. Chemical reactions involve the release or. Error In Calorimetry Experiment.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Error In Calorimetry Experiment Chemical reactions involve the release or consumption of energy, usually. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure. It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. if δh is. Error In Calorimetry Experiment.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Error In Calorimetry Experiment if δh is negative, the reaction is exothermic. have a chemistry practical on the calorimetry experiment? Learn how to perform and analyse the calorimetry practical and ace your next chemistry practical assessment. If δh is positive, the reaction is endothermic. Calorimetry is used to measure. in this experiment you will heat a known mass of a metal. Error In Calorimetry Experiment.
From www.numerade.com
SOLVED Method Error Calorimeter Heat loss from the calorimeter is Error In Calorimetry Experiment Learn how to perform and analyse the calorimetry practical and ace your next chemistry practical assessment. Calorimetry is used to measure. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. if δh is negative, the reaction is exothermic. It is impossible to obtain an exact. Error In Calorimetry Experiment.
From www.slideshare.net
4 Calorimetry Error In Calorimetry Experiment If δh is positive, the reaction is endothermic. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. The heat of the reaction cannot be. the calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. one technique. Error In Calorimetry Experiment.
From www.researchgate.net
(PDF) Propagation of uncertainties and systematic errors in the Error In Calorimetry Experiment If δh is positive, the reaction is endothermic. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. have a chemistry practical on the calorimetry experiment? if δh is negative, the reaction is exothermic. the calorimeter constant ccal calorimetry is the science of measuring. Error In Calorimetry Experiment.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Error In Calorimetry Experiment If δh is positive, the reaction is endothermic. have a chemistry practical on the calorimetry experiment? in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. Calorimetry is used to measure. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry. Error In Calorimetry Experiment.
From studylib.net
Calorimetry Experiment and Practice Problems for Virtual Lab Key Error In Calorimetry Experiment It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. if δh is negative, the reaction is exothermic. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. have a chemistry practical on the calorimetry experiment? Learn. Error In Calorimetry Experiment.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Error In Calorimetry Experiment If δh is positive, the reaction is endothermic. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The heat of the reaction cannot be. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. in. Error In Calorimetry Experiment.
From www.nagwa.com
Question Video Identifying the Factor That Does Not Need to Be Held Error In Calorimetry Experiment If δh is positive, the reaction is endothermic. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. Calorimetry is used to measure. It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. if δh is negative, the. Error In Calorimetry Experiment.
From tukioka-clinic.com
😂 Soda can calorimeter sources of error. What Is a Calorimeter & What Error In Calorimetry Experiment The heat of the reaction cannot be. revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. have a chemistry practical on the calorimetry experiment? . Error In Calorimetry Experiment.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Error In Calorimetry Experiment one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. the calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. if δh is negative, the reaction is exothermic. It is impossible to obtain an. Error In Calorimetry Experiment.
From answermagicmatney.z21.web.core.windows.net
Coffee Cup Calorimetry Experiment Error In Calorimetry Experiment It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. Chemical reactions involve the release or consumption of energy, usually. if δh is negative, the reaction is exothermic. the calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. . Error In Calorimetry Experiment.
From www.youtube.com
Calorimetry Problem Solving YouTube Error In Calorimetry Experiment the calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. Calorimetry is used to measure. Chemical reactions involve the release or consumption of energy, usually. if δh is negative, the reaction is exothermic. It is impossible to obtain an exact measurement due to the lack of precision. Error In Calorimetry Experiment.
From www.youtube.com
CHEM 1411 Calorimetry Experiment YouTube Error In Calorimetry Experiment if δh is negative, the reaction is exothermic. the calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. Chemical reactions involve the release or. Error In Calorimetry Experiment.
From www.coursehero.com
[Solved] QUESTION 44 A chemist conducts a calorimetry experiment on an Error In Calorimetry Experiment Learn how to perform and analyse the calorimetry practical and ace your next chemistry practical assessment. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. have a chemistry practical on the calorimetry experiment? The heat of the reaction cannot be. If δh is positive, the. Error In Calorimetry Experiment.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Error In Calorimetry Experiment revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. have a chemistry practical on the calorimetry experiment? If δh is positive, the reaction is endothermic. the calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. Chemical. Error In Calorimetry Experiment.
From www.slideserve.com
PPT Energy in food PowerPoint Presentation, free download ID5830775 Error In Calorimetry Experiment the calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. in this experiment you will heat a known mass of a metal to a. Error In Calorimetry Experiment.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Error In Calorimetry Experiment revision notes on calorimetry for the edexcel igcse chemistry syllabus, written by the chemistry experts at save my exams. It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. Calorimetry is used to measure. If δh is positive, the reaction is endothermic. Learn how to perform and analyse the calorimetry practical. Error In Calorimetry Experiment.
From learningcampusfox.z1.web.core.windows.net
Coffee Cup Calorimetry Practice Problems Error In Calorimetry Experiment It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. the calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. if δh is negative, the reaction is exothermic. one technique we can use to measure the amount of. Error In Calorimetry Experiment.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Error In Calorimetry Experiment Chemical reactions involve the release or consumption of energy, usually. Calorimetry is used to measure. The heat of the reaction cannot be. have a chemistry practical on the calorimetry experiment? It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. the calorimeter constant ccal calorimetry is the science of measuring. Error In Calorimetry Experiment.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Error In Calorimetry Experiment in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. if δh is negative, the reaction is exothermic. The heat of the reaction cannot be. Learn how to perform and analyse the calorimetry practical and ace your next chemistry practical assessment. If δh is positive, the. Error In Calorimetry Experiment.
From www.numerade.com
SOLVED Name three possible sources of error in a calorimetry experiment. Error In Calorimetry Experiment have a chemistry practical on the calorimetry experiment? The heat of the reaction cannot be. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. revision notes. Error In Calorimetry Experiment.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Error In Calorimetry Experiment the calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. if δh is negative, the reaction is exothermic. have a chemistry practical on the calorimetry experiment? It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. Learn how. Error In Calorimetry Experiment.
From www.researchgate.net
Sources of error in calorimetry measurements Download Scientific Diagram Error In Calorimetry Experiment have a chemistry practical on the calorimetry experiment? one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. The heat of the reaction cannot be. the calorimeter. Error In Calorimetry Experiment.
From philschatz.com
Calorimetry · Chemistry Error In Calorimetry Experiment if δh is negative, the reaction is exothermic. Calorimetry is used to measure. in this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a. If δh is positive, the reaction is endothermic. It is impossible to obtain an exact measurement due to the lack of precision of. Error In Calorimetry Experiment.
From studylib.net
5.1 b) CALORIMETRY Error In Calorimetry Experiment It is impossible to obtain an exact measurement due to the lack of precision of instruments and the. one technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Chemical reactions involve the release or consumption of energy, usually. The heat of the reaction cannot be. Calorimetry is. Error In Calorimetry Experiment.
From www.youtube.com
Effects of experimental errors on enthalpy measurements YouTube Error In Calorimetry Experiment Learn how to perform and analyse the calorimetry practical and ace your next chemistry practical assessment. If δh is positive, the reaction is endothermic. Calorimetry is used to measure. the calorimeter constant ccal calorimetry is the science of measuring the quantities of heat released or absorbed during a chemical reaction. The heat of the reaction cannot be. if. Error In Calorimetry Experiment.