Is Thermal Energy Kinetic . Is it the size of the particles or number of. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic. learn about thermal energy, how it is transferred, and how it affects the state of matter. Khan academy offers free, world. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. what is the difference between kinetic energy and thermal energy?
from www.slideserve.com
Khan academy offers free, world. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. what is the difference between kinetic energy and thermal energy? Is it the size of the particles or number of. learn about thermal energy, how it is transferred, and how it affects the state of matter. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic.
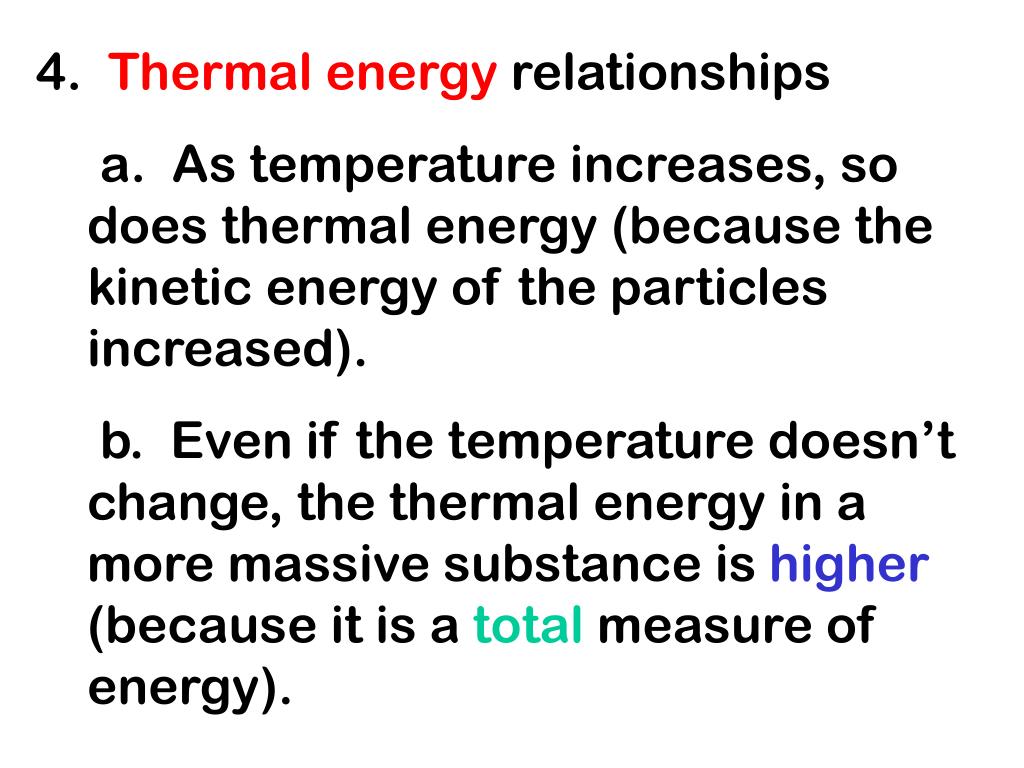
PPT Thermal Energy A. Temperature & Heat 1. Temperature is related to
Is Thermal Energy Kinetic Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. what is the difference between kinetic energy and thermal energy? without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic. learn about thermal energy, how it is transferred, and how it affects the state of matter. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. Khan academy offers free, world. Is it the size of the particles or number of. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium.
From www.slideshare.net
Thermal Energy And You Is Thermal Energy Kinetic without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. Is it the size of the particles or number of. Khan academy offers free, world. thermal energy. Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Chapter 10 Thermal Physics PowerPoint Presentation, free download Is Thermal Energy Kinetic Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. Is it the size of the particles or number of. learn about thermal energy, how it is transferred, and how it affects the state of matter. what is the difference between kinetic energy and thermal. Is Thermal Energy Kinetic.
From studylib.net
Thermal Energy and Heat Transfer Thermal Energy Molecular Theory Is Thermal Energy Kinetic Is it the size of the particles or number of. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. Khan academy offers free, world. learn about thermal energy, how it is transferred, and how it affects the state of matter. what is the difference. Is Thermal Energy Kinetic.
From briesnitzhnschematic.z14.web.core.windows.net
What Happens When Thermal Energy Increases Is Thermal Energy Kinetic learn about thermal energy, how it is transferred, and how it affects the state of matter. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. . Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Heat PowerPoint Presentation, free download ID2977447 Is Thermal Energy Kinetic Khan academy offers free, world. thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic. learn about thermal energy, how it is transferred, and how it affects the state of matter. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average.. Is Thermal Energy Kinetic.
From www.slideshare.net
Lesson 1 thermal energy Is Thermal Energy Kinetic learn about thermal energy, how it is transferred, and how it affects the state of matter. Is it the size of the particles or number of. Khan academy offers free, world. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. thermal energy, internal energy present in a system. Is Thermal Energy Kinetic.
From studylib.net
Chpt 6 Thermal Energy Is Thermal Energy Kinetic what is the difference between kinetic energy and thermal energy? thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. Thermal energy cannot be converted to useful work as easily. Is Thermal Energy Kinetic.
From slideplayer.com
Thermal Energy and Heat ppt download Is Thermal Energy Kinetic Khan academy offers free, world. Is it the size of the particles or number of. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. what is the difference between kinetic energy and thermal energy? learn about thermal energy, how it is transferred, and how it affects the. Is Thermal Energy Kinetic.
From dokumen.tips
(PPTX) Heat Chapter 9 Thermal Energy. and Potential Energy Is Thermal Energy Kinetic learn about thermal energy, how it is transferred, and how it affects the state of matter. what is the difference between kinetic energy and thermal energy? Is it the size of the particles or number of. Khan academy offers free, world. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is. Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Thermal Energy A. Temperature & Heat 1. Temperature is related to Is Thermal Energy Kinetic thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. Is it the size of the particles or number of. learn about thermal energy, how it is transferred, and how. Is Thermal Energy Kinetic.
From exorkgevk.blob.core.windows.net
Can Energy Be Converted To Thermal Energy at John Sollars blog Is Thermal Energy Kinetic what is the difference between kinetic energy and thermal energy? Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. learn about thermal energy, how it is transferred, and how it affects the state of matter. without going into mathematical detail, we can say. Is Thermal Energy Kinetic.
From lessoncampuspolypide.z5.web.core.windows.net
Potential And Energy Video 5th Grade Is Thermal Energy Kinetic learn about thermal energy, how it is transferred, and how it affects the state of matter. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature.. Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Temperature measures the average energy of PowerPoint Is Thermal Energy Kinetic thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic. Is Thermal Energy Kinetic.
From quizlet.com
Thermal, and Potential Energy Diagram Quizlet Is Thermal Energy Kinetic Is it the size of the particles or number of. Khan academy offers free, world. learn about thermal energy, how it is transferred, and how it affects the state of matter. thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic. what is the difference between kinetic energy and. Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Unit 6 PowerPoint Presentation, free download ID1516058 Is Thermal Energy Kinetic Khan academy offers free, world. learn about thermal energy, how it is transferred, and how it affects the state of matter. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. what is the difference between kinetic energy and thermal energy? without going into mathematical detail, we. Is Thermal Energy Kinetic.
From thepowerfacts.com
Is Heat Energy Potential Or (Answer is Here) The Power Facts Is Thermal Energy Kinetic learn about thermal energy, how it is transferred, and how it affects the state of matter. Is it the size of the particles or number of. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. what is the difference between kinetic energy and thermal energy? thermal energy,. Is Thermal Energy Kinetic.
From www.slideserve.com
PPT How to Use This Presentation PowerPoint Presentation, free Is Thermal Energy Kinetic Khan academy offers free, world. thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. Is it the size of the particles or number of. Thermal energy cannot be converted to useful. Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Heat and Thermal Energy PowerPoint Presentation, free download Is Thermal Energy Kinetic what is the difference between kinetic energy and thermal energy? without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. Thermal energy cannot be converted to useful work as easily. Is Thermal Energy Kinetic.
From www.pinterest.co.uk
When surfaces in contact move relative to each other, the friction Is Thermal Energy Kinetic Khan academy offers free, world. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. Is it the size of the particles or number of. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. thermal energy is the total. Is Thermal Energy Kinetic.
From sciencing.com
Thermal Energy Definition, Equation, Types (w/ Diagram & Examples Is Thermal Energy Kinetic Khan academy offers free, world. thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. Is it the size of the particles or number of. Thermal energy cannot be converted to useful. Is Thermal Energy Kinetic.
From www.vecteezy.com
Energy and Temperature science vector graphic 21790106 Vector Is Thermal Energy Kinetic what is the difference between kinetic energy and thermal energy? without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. Thermal energy cannot be converted to useful work as easily. Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Is Thermal Energy Kinetic thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic. Khan academy offers free, world. without going into mathematical detail, we can say that thermal energy —the energy associated with. Is Thermal Energy Kinetic.
From webapi.bu.edu
💌 Thermal energy energy. What Are the Differences Between Is Thermal Energy Kinetic Is it the size of the particles or number of. thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. Thermal energy cannot be converted to useful work as easily as the. Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Thermal Physics PowerPoint Presentation, free download ID5760025 Is Thermal Energy Kinetic what is the difference between kinetic energy and thermal energy? thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic. learn about thermal energy, how it is transferred, and how it affects the state of matter. without going into mathematical detail, we can say that thermal energy —the. Is Thermal Energy Kinetic.
From haipernews.com
How To Calculate Energy Thermal Haiper Is Thermal Energy Kinetic Is it the size of the particles or number of. Khan academy offers free, world. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. without. Is Thermal Energy Kinetic.
From www.sciencefacts.net
Thermal (Heat) Energy Definition, Examples, Equations, and Units Is Thermal Energy Kinetic what is the difference between kinetic energy and thermal energy? thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. Thermal energy cannot be converted to useful work as easily. Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Thermal Energy A. Temperature & Heat 1. Temperature is related to Is Thermal Energy Kinetic thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. Is it the size of the particles or number of. Khan academy offers free, world. without. Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Thermal Energy PowerPoint Presentation, free download ID4251421 Is Thermal Energy Kinetic thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. Is it the size of the particles or number of. what is the difference between kinetic. Is Thermal Energy Kinetic.
From steamism.com
The 2 types and 9 forms of Energy and Potential Is Thermal Energy Kinetic thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic. learn about thermal energy, how it is transferred, and how it affects the state of matter. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average. Is it the size of. Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Heat, Thermodynamics, Heat Transfer and Weather PowerPoint Is Thermal Energy Kinetic Khan academy offers free, world. learn about thermal energy, how it is transferred, and how it affects the state of matter. thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the average.. Is Thermal Energy Kinetic.
From sites.google.com
Energy Mrs. Sanborn's Site Is Thermal Energy Kinetic Khan academy offers free, world. Is it the size of the particles or number of. learn about thermal energy, how it is transferred, and how it affects the state of matter. thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. what is the difference between kinetic energy. Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Chapter 6 Thermochemistry PowerPoint Presentation, free download Is Thermal Energy Kinetic what is the difference between kinetic energy and thermal energy? Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic. thermal energy, internal energy present in. Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Energy and Matter PowerPoint Presentation, free download ID6834725 Is Thermal Energy Kinetic Is it the size of the particles or number of. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic. thermal energy, internal energy present in a. Is Thermal Energy Kinetic.
From study.com
Thermal Energy Definition & Examples Lesson Is Thermal Energy Kinetic thermal energy, internal energy present in a system in a state of thermodynamic equilibrium by virtue of its temperature. learn about thermal energy, how it is transferred, and how it affects the state of matter. thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic. without going into. Is Thermal Energy Kinetic.
From www.slideserve.com
PPT Thermal Physics PowerPoint Presentation, free download ID5760025 Is Thermal Energy Kinetic thermal energy is the total kinetic energy within a system, observed as either vibrational, rotational or translational kinetic. Thermal energy cannot be converted to useful work as easily as the energy of systems that are not in states of thermodynamic equilibrium. without going into mathematical detail, we can say that thermal energy —the energy associated with heat—is the. Is Thermal Energy Kinetic.