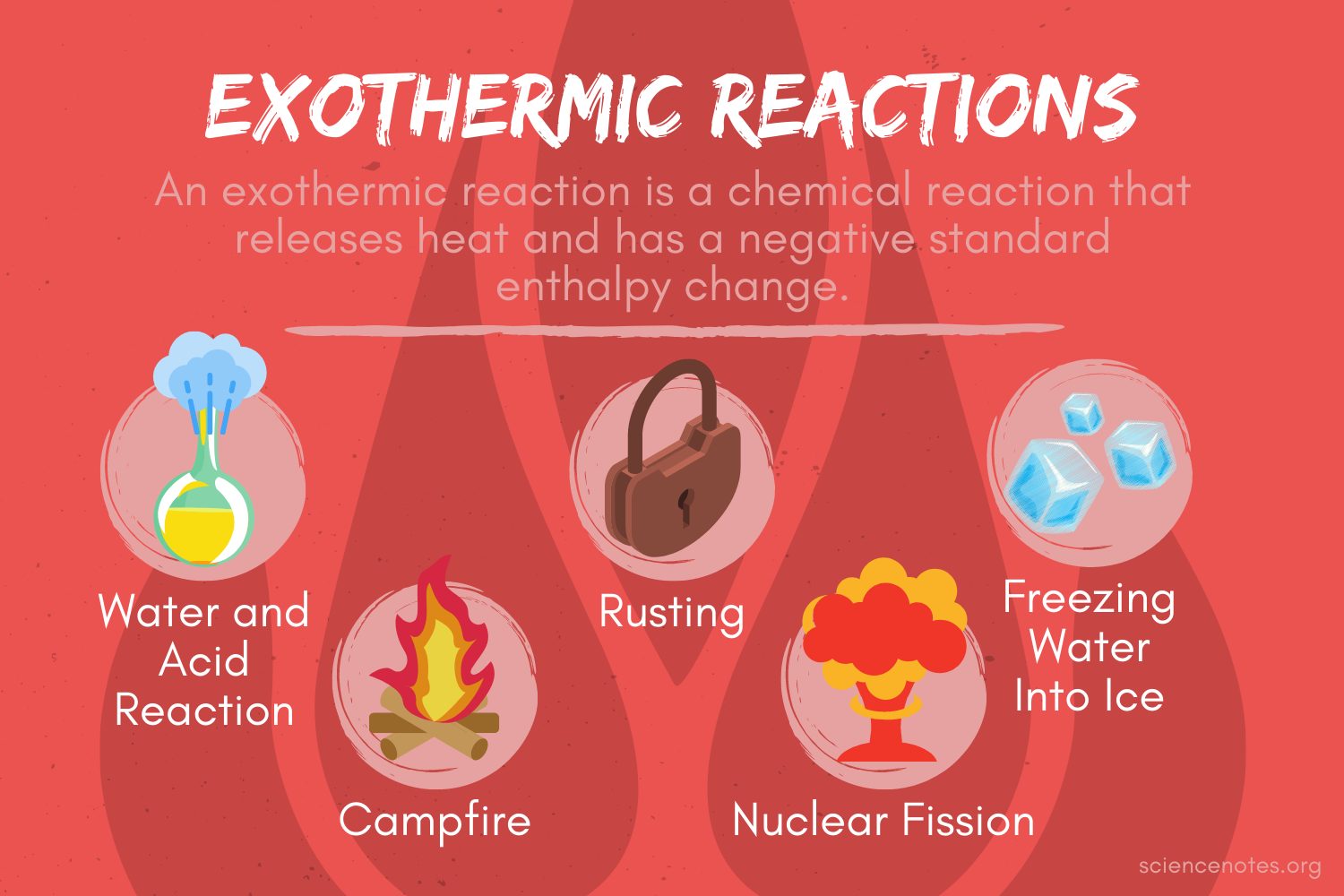
Is Phase Change Endothermic Or Exothermic . changes of state are examples of phase changes, or phase transitions. A reaction that releases the same amount of energy as is put in is called an. a phase change is a physical process in which a substance goes from one phase to another. An endothermic process absorbs heat and cools the surroundings.” based on the above definition, let's pick a. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. All phase changes are accompanied by changes in the energy of a system. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. it's a great resource if you are struggling with understanding which. Changes of state are examples of phase. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes.
from classnotes123.com
a phase change is a physical process in which a substance goes from one phase to another. All phase changes are accompanied by changes in the energy of a system. An endothermic process absorbs heat and cools the surroundings.” based on the above definition, let's pick a. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Changes of state are examples of phase. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. A reaction that releases the same amount of energy as is put in is called an. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. changes of state are examples of phase changes, or phase transitions. it's a great resource if you are struggling with understanding which.
What does one mean by exothermic and endothermic reactions? Give
Is Phase Change Endothermic Or Exothermic it's a great resource if you are struggling with understanding which. a phase change is a physical process in which a substance goes from one phase to another. A reaction that releases the same amount of energy as is put in is called an. Changes of state are examples of phase. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. it's a great resource if you are struggling with understanding which. All phase changes are accompanied by changes in the energy of a system. changes of state are examples of phase changes, or phase transitions. An endothermic process absorbs heat and cools the surroundings.” based on the above definition, let's pick a. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or.
From stock.adobe.com
Activation energy in endothermic and exothermic reactions. Stock Is Phase Change Endothermic Or Exothermic Changes of state are examples of phase. A reaction that releases the same amount of energy as is put in is called an. An endothermic process absorbs heat and cools the surroundings.” based on the above definition, let's pick a. changes of state are examples of phase changes, or phase transitions. Usually the change occurs when adding or removing. Is Phase Change Endothermic Or Exothermic.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Is Phase Change Endothermic Or Exothermic changes of state are examples of phase changes, or phase transitions. All phase changes are accompanied by changes in the energy of a system. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. Changes of state are examples. Is Phase Change Endothermic Or Exothermic.
From catholicscienceteacher8.blogspot.com
Mrs. Remis' Science Blog 8th grade ENDOTHERMIC & EXOTHERMIC PROCESS Is Phase Change Endothermic Or Exothermic A reaction that releases the same amount of energy as is put in is called an. changes of state are examples of phase changes, or phase transitions. a phase change is a physical process in which a substance goes from one phase to another. An endothermic process absorbs heat and cools the surroundings.” based on the above definition,. Is Phase Change Endothermic Or Exothermic.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Is Phase Change Endothermic Or Exothermic Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. it's a great resource if you are struggling with understanding which. All phase changes are accompanied by changes in the energy of a system. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat. Is Phase Change Endothermic Or Exothermic.
From www.youtube.com
Lecture 1.11 exothermic, endothermic, phase changes, YouTube Is Phase Change Endothermic Or Exothermic an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. it's a great resource if you are struggling with understanding which. A reaction that releases the same amount of energy as is put in is called an. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting. Is Phase Change Endothermic Or Exothermic.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! Is Phase Change Endothermic Or Exothermic a phase change is a physical process in which a substance goes from one phase to another. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. Changes of state are examples of phase. A reaction that releases the same amount of energy as is put in is called an.. Is Phase Change Endothermic Or Exothermic.
From sciencing.com
What Phase Changes Are Exothermic & Endothermic? Sciencing Is Phase Change Endothermic Or Exothermic A reaction that releases the same amount of energy as is put in is called an. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. An endothermic process absorbs heat and cools the surroundings.” based on the above definition, let's pick a. a phase change is a physical process in which a substance. Is Phase Change Endothermic Or Exothermic.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID1756244 Is Phase Change Endothermic Or Exothermic it's a great resource if you are struggling with understanding which. a phase change is a physical process in which a substance goes from one phase to another. An endothermic process absorbs heat and cools the surroundings.” based on the above definition, let's pick a. A reaction that releases the same amount of energy as is put in. Is Phase Change Endothermic Or Exothermic.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Is Phase Change Endothermic Or Exothermic An endothermic process absorbs heat and cools the surroundings.” based on the above definition, let's pick a. a phase change is a physical process in which a substance goes from one phase to another. Changes of state are examples of phase. it's a great resource if you are struggling with understanding which. All phase changes are accompanied by. Is Phase Change Endothermic Or Exothermic.
From opentextbc.ca
Phase Changes Basic HVAC Is Phase Change Endothermic Or Exothermic Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. it's a great resource if you are struggling with understanding which. Changes of state are examples of phase. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. an exothermic process releases heat, causing. Is Phase Change Endothermic Or Exothermic.
From www.researchgate.net
DSC curves of endothermic and exothermic phase changes during a heating Is Phase Change Endothermic Or Exothermic a phase change is a physical process in which a substance goes from one phase to another. Changes of state are examples of phase. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. changes of state are examples of phase changes, or phase transitions. An endothermic process absorbs heat and cools the. Is Phase Change Endothermic Or Exothermic.
From proper-cooking.info
Endothermic And Exothermic Phase Changes Is Phase Change Endothermic Or Exothermic Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. All phase changes are accompanied by changes in the energy of a system. A reaction that releases the same amount of energy as is put in is called an. An. Is Phase Change Endothermic Or Exothermic.
From lacorrala.online
Endotherm Exotherm Diagramm Unterschied Zwischen Und Homeotherm Is Phase Change Endothermic Or Exothermic changes of state are examples of phase changes, or phase transitions. An endothermic process absorbs heat and cools the surroundings.” based on the above definition, let's pick a. A reaction that releases the same amount of energy as is put in is called an. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise.. Is Phase Change Endothermic Or Exothermic.
From www.pinterest.com
Login to Your BenchPrep Account Teaching chemistry, Chemistry Is Phase Change Endothermic Or Exothermic An endothermic process absorbs heat and cools the surroundings.” based on the above definition, let's pick a. changes of state are examples of phase changes, or phase transitions. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. All phase changes are accompanied by changes in the energy of a. Is Phase Change Endothermic Or Exothermic.
From quizizz.com
Endothermic vs Exothermic Process Chemistry Quiz Quizizz Is Phase Change Endothermic Or Exothermic Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. changes of state are examples of phase changes, or phase transitions. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. Changes of state are examples of phase. A reaction that releases the same amount of energy as is. Is Phase Change Endothermic Or Exothermic.
From kr.freepik.com
흡열 및 발열 반응의 활성화 에너지 프리미엄 벡터 Is Phase Change Endothermic Or Exothermic A reaction that releases the same amount of energy as is put in is called an. All phase changes are accompanied by changes in the energy of a system. a phase change is a physical process in which a substance goes from one phase to another. An endothermic process absorbs heat and cools the surroundings.” based on the above. Is Phase Change Endothermic Or Exothermic.
From maxim-kwise.blogspot.com
The Process by Which a Gas Changes Into a Liquid Is Phase Change Endothermic Or Exothermic Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. it's a great resource if you are struggling with understanding which. a phase change is a physical process in which a substance goes from. Is Phase Change Endothermic Or Exothermic.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Is Phase Change Endothermic Or Exothermic All phase changes are accompanied by changes in the energy of a system. An endothermic process absorbs heat and cools the surroundings.” based on the above definition, let's pick a. changes of state are examples of phase changes, or phase transitions. Changes of state are examples of phase. a phase change is a physical process in which a. Is Phase Change Endothermic Or Exothermic.
From alwafd.org
Endothermic Reaction Definition, Equation, Graph & Examples What Is Phase Change Endothermic Or Exothermic an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. a phase change is a physical process in which a substance goes from one phase to another. A reaction that releases the same amount of energy as is put in is called an. Usually the change occurs when adding or removing heat at a. Is Phase Change Endothermic Or Exothermic.
From stock.adobe.com
Vector graphs or charts of endothermic and exothermic reactions Is Phase Change Endothermic Or Exothermic Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. Changes of state are examples of phase. changes of state are examples of phase changes, or phase transitions. it's a great resource if you. Is Phase Change Endothermic Or Exothermic.
From ar.inspiredpencil.com
Endothermic And Exothermic Reactions Is Phase Change Endothermic Or Exothermic Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. An endothermic process absorbs heat and cools the surroundings.” based on the above definition, let's pick a. All phase changes are accompanied by changes in the energy of a system. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and. Is Phase Change Endothermic Or Exothermic.
From www.sexizpix.com
Ppt Endothermic Vs Exothermic Reaction Graphs Powerpoint Sexiz Pix Is Phase Change Endothermic Or Exothermic A reaction that releases the same amount of energy as is put in is called an. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. Changes of state are examples of phase. An endothermic process absorbs heat and cools the surroundings.” based on the above definition, let's pick a. changes of state are. Is Phase Change Endothermic Or Exothermic.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Is Phase Change Endothermic Or Exothermic All phase changes are accompanied by changes in the energy of a system. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. it's a great resource if you are struggling with understanding which. changes of state are examples of phase changes, or phase transitions. Usually the change occurs when adding or removing. Is Phase Change Endothermic Or Exothermic.
From sciencing.com
What Phase Changes Are Exothermic & Endothermic? Sciencing Is Phase Change Endothermic Or Exothermic changes of state are examples of phase changes, or phase transitions. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. it's a great resource if you are struggling with understanding which. a. Is Phase Change Endothermic Or Exothermic.
From www.researchgate.net
Schematic illustration of endothermic and exothermic phases. Download Is Phase Change Endothermic Or Exothermic Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. changes of state are examples of phase changes, or phase transitions. a phase change is a physical process in which a substance goes from one phase to another.. Is Phase Change Endothermic Or Exothermic.
From www.slideserve.com
PPT ENTHALPY OF FORMATION Combustion of Methanol PowerPoint Is Phase Change Endothermic Or Exothermic a phase change is a physical process in which a substance goes from one phase to another. An endothermic process absorbs heat and cools the surroundings.” based on the above definition, let's pick a. A reaction that releases the same amount of energy as is put in is called an. Usually the change occurs when adding or removing heat. Is Phase Change Endothermic Or Exothermic.
From brainly.com
draw an energy diagram for an endothermic and exothermic reaction and Is Phase Change Endothermic Or Exothermic an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. Changes of state are examples of phase. it's a great resource if you are struggling with understanding which. All phase changes are accompanied by changes in the energy of a system. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are. Is Phase Change Endothermic Or Exothermic.
From www.fity.club
Comparing Endothermic And Exothermic Potential Energy Is Phase Change Endothermic Or Exothermic A reaction that releases the same amount of energy as is put in is called an. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. it's a great resource if you are struggling with understanding which. Usually the. Is Phase Change Endothermic Or Exothermic.
From www.youtube.com
Endothermic and exothermic reactions. Enthalpy YouTube Is Phase Change Endothermic Or Exothermic A reaction that releases the same amount of energy as is put in is called an. changes of state are examples of phase changes, or phase transitions. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. All phase changes are accompanied by changes in the energy of a system. Usually the change occurs. Is Phase Change Endothermic Or Exothermic.
From chhattisgarh.pscnotes.com
Exothermic and endothermic reactions CGPCS Exam Preparation Is Phase Change Endothermic Or Exothermic Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. Fusion, vaporization, and sublimation are endothermic processes, whereas freezing, condensation, and deposition are exothermic processes. Changes of state are examples of phase. All phase changes are accompanied by changes in the energy of a system. an exothermic process releases heat,. Is Phase Change Endothermic Or Exothermic.
From www.youtube.com
How to Determine if Phase Change is Endothermic or Exothermic Examples Is Phase Change Endothermic Or Exothermic A reaction that releases the same amount of energy as is put in is called an. it's a great resource if you are struggling with understanding which. An endothermic process absorbs heat and cools the surroundings.” based on the above definition, let's pick a. All phase changes are accompanied by changes in the energy of a system. a. Is Phase Change Endothermic Or Exothermic.
From www.aiophotoz.com
Endothermic Reaction Diagram Labeled Diagram Media Images and Photos Is Phase Change Endothermic Or Exothermic A reaction that releases the same amount of energy as is put in is called an. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. An endothermic process absorbs heat and cools the surroundings.” based on the above definition, let's pick a. Changes of state are examples of phase. . Is Phase Change Endothermic Or Exothermic.
From www.adda247.com
Endothermic Reaction Examples, Equations, Formula for Class 10 Is Phase Change Endothermic Or Exothermic it's a great resource if you are struggling with understanding which. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or. a phase change is a physical process in which a substance goes from one phase to another. an exothermic process releases heat, causing the temperature of the. Is Phase Change Endothermic Or Exothermic.
From worksheets.ekocraft-appleleaf.com
Endothermic Vs Exothermic Worksheet Worksheets For Kindergarten Is Phase Change Endothermic Or Exothermic Changes of state are examples of phase. A reaction that releases the same amount of energy as is put in is called an. a phase change is a physical process in which a substance goes from one phase to another. Usually the change occurs when adding or removing heat at a particular temperature, known as the melting point or.. Is Phase Change Endothermic Or Exothermic.
From www.researchgate.net
Endothermic or exothermic reaction of the VO 2 phase caused by phase Is Phase Change Endothermic Or Exothermic A reaction that releases the same amount of energy as is put in is called an. an exothermic process releases heat, causing the temperature of the immediate surroundings to rise. changes of state are examples of phase changes, or phase transitions. Changes of state are examples of phase. An endothermic process absorbs heat and cools the surroundings.” based. Is Phase Change Endothermic Or Exothermic.