Can Hydrogen Form A Negative Ion . In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. As you might expect, this ion is very weakly bound together—in fact,. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. The formation of such an ion can be qualitatively described, in. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion.
from www.expii.com
In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. As you might expect, this ion is very weakly bound together—in fact,. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. The formation of such an ion can be qualitatively described, in.
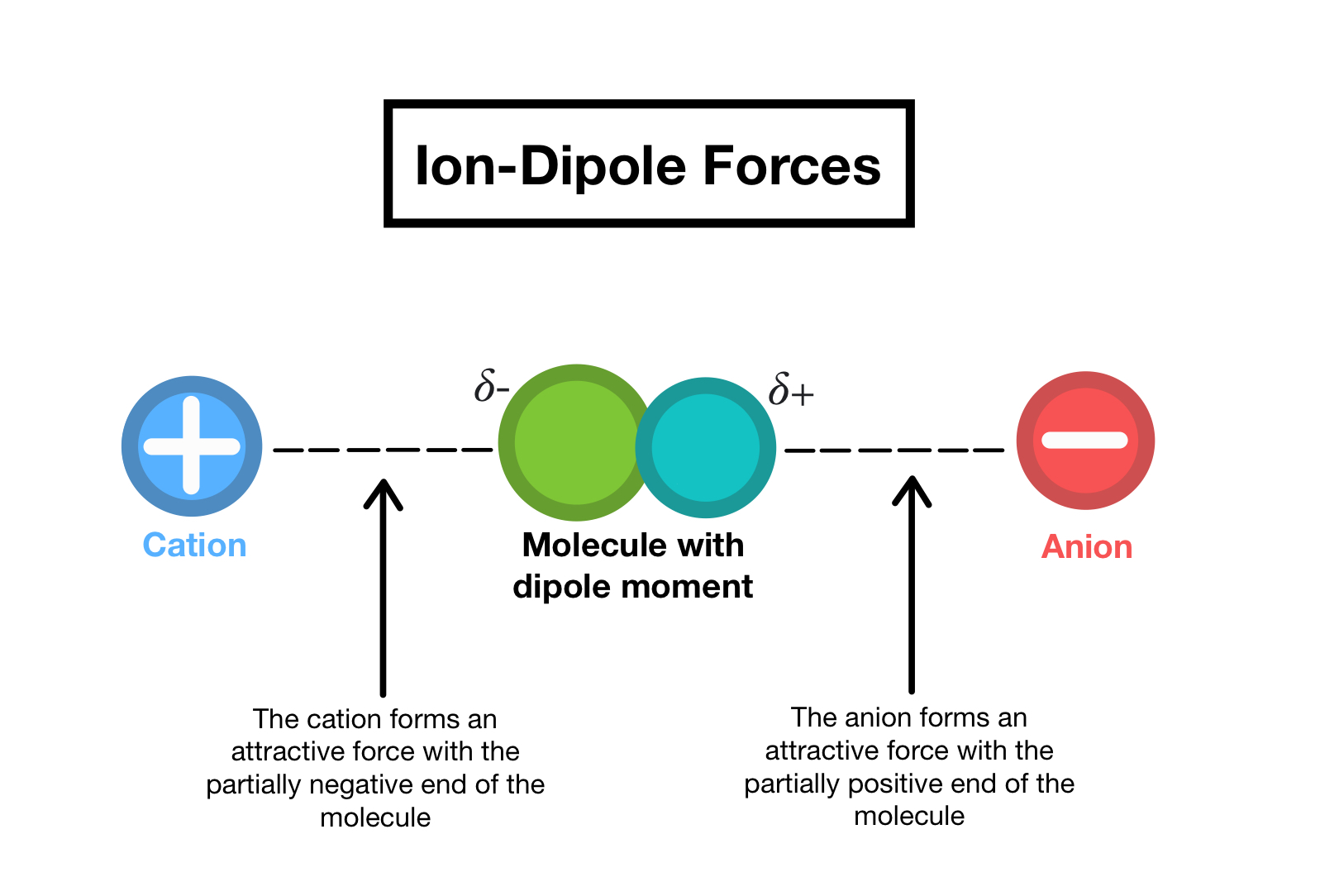
IonDipole Forces — Definition & Overview Expii
Can Hydrogen Form A Negative Ion The formation of such an ion can be qualitatively described, in. As you might expect, this ion is very weakly bound together—in fact,. The formation of such an ion can be qualitatively described, in. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons.
From www.teachoo.com
In which year is concentration of hydrogen ion highest? MCQ Class 10 Can Hydrogen Form A Negative Ion Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. The. Can Hydrogen Form A Negative Ion.
From byjus.com
In water, oxygen has a slight negative charge and hydrogen has a slight Can Hydrogen Form A Negative Ion The formation of such an ion can be qualitatively described, in. As you might expect, this ion is very weakly bound together—in fact,. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and. Can Hydrogen Form A Negative Ion.
From qnet-india.in
Negative Hydrogen (H) The Antioxidant Superhero! Can Hydrogen Form A Negative Ion The formation of such an ion can be qualitatively described, in. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. In this section, we are interested in the negative hydrogen ion, h− h −,. Can Hydrogen Form A Negative Ion.
From www.numerade.com
Deduce the concentration of hydrogen ions (H+) and hydroxide ions (OH Can Hydrogen Form A Negative Ion Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. As you might expect, this ion is very weakly bound together—in fact,. The formation of such an ion can be qualitatively described, in. In this. Can Hydrogen Form A Negative Ion.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning Can Hydrogen Form A Negative Ion In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. The formation of such an ion can be qualitatively described, in. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. As you might expect, this. Can Hydrogen Form A Negative Ion.
From www.researchgate.net
Hydrolysis of water forms hydrogen ions at the positive electrode and Can Hydrogen Form A Negative Ion As you might expect, this ion is very weakly bound together—in fact,. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. Of course, elements with medium levels of electronegativity (such. Can Hydrogen Form A Negative Ion.
From material-properties.org
Hydrogen Periodic Table and Atomic Properties Can Hydrogen Form A Negative Ion Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. As you might expect, this ion is very weakly bound together—in fact,. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. The formation of such an ion can be qualitatively. Can Hydrogen Form A Negative Ion.
From quizlet.com
hydrogen bond between water molecules Diagram Quizlet Can Hydrogen Form A Negative Ion As you might expect, this ion is very weakly bound together—in fact,. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. Of course, elements with medium levels of electronegativity (such. Can Hydrogen Form A Negative Ion.
From spmchemistry.blog.onlinetuition.com.my
Formation of Negative Ions SPM Chemistry Can Hydrogen Form A Negative Ion Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. As you might expect, this ion is very weakly bound together—in fact,. The formation of such an ion can be qualitatively described, in. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and. Can Hydrogen Form A Negative Ion.
From www.pinterest.com.au
Water has both a hydrogen bond and a polar covalent bond. Hydrogen Can Hydrogen Form A Negative Ion As you might expect, this ion is very weakly bound together—in fact,. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. The formation of such. Can Hydrogen Form A Negative Ion.
From www.thesciencehive.co.uk
Ionic Bonding — the science sauce Can Hydrogen Form A Negative Ion In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. As. Can Hydrogen Form A Negative Ion.
From ar.inspiredpencil.com
Water Molecules Hydrogen Bonds Can Hydrogen Form A Negative Ion In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. As you might expect, this ion is very weakly bound together—in fact,. The formation of such an ion can be qualitatively described, in. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon). Can Hydrogen Form A Negative Ion.
From socratic.org
Two hydrogen atoms interact to form a hydrogen molecule. Classify the Can Hydrogen Form A Negative Ion In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. As you might expect, this ion is very weakly bound together—in fact,. Hydrogen is highly polarized,. Can Hydrogen Form A Negative Ion.
From www.youtube.com
Can hydrogen form ionic bond? YouTube Can Hydrogen Form A Negative Ion In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. As you might expect, this ion is very weakly bound together—in fact,. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. The formation of such. Can Hydrogen Form A Negative Ion.
From mungfali.com
Hydrogen Atomic Structure Can Hydrogen Form A Negative Ion The formation of such an ion can be qualitatively described, in. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. In this section, we are interested in the negative hydrogen ion, h− h −,. Can Hydrogen Form A Negative Ion.
From www.eurekalert.org
Simplified Image of Negative H [IMAGE] EurekAlert! Science News Releases Can Hydrogen Form A Negative Ion Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. As you might expect, this ion is very weakly bound together—in fact,. Of course, elements with medium levels of electronegativity (such. Can Hydrogen Form A Negative Ion.
From www.slideserve.com
PPT Ion Formation PowerPoint Presentation ID2508414 Can Hydrogen Form A Negative Ion The formation of such an ion can be qualitatively described, in. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. In this section, we are interested in the negative hydrogen ion, h− h −,. Can Hydrogen Form A Negative Ion.
From courses.lumenlearning.com
Chemical Bonds Anatomy and Physiology I Can Hydrogen Form A Negative Ion As you might expect, this ion is very weakly bound together—in fact,. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. Of course, elements with medium levels of electronegativity (such. Can Hydrogen Form A Negative Ion.
From slidetodoc.com
Ionic Bonding Elements are the simplest substances There Can Hydrogen Form A Negative Ion Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. The formation of such an ion can be qualitatively described, in. Of course, elements with medium levels of electronegativity (such as. Can Hydrogen Form A Negative Ion.
From hebasoffar.blogspot.com
Science online The electronic configuration and the chemical activity Can Hydrogen Form A Negative Ion The formation of such an ion can be qualitatively described, in. As you might expect, this ion is very weakly bound together—in fact,. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. In this. Can Hydrogen Form A Negative Ion.
From molecularhydrogeninstitute.org
Dummies Guide To Hydrogen MHI Can Hydrogen Form A Negative Ion The formation of such an ion can be qualitatively described, in. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. As you might expect, this ion is very weakly bound together—in fact,. In this. Can Hydrogen Form A Negative Ion.
From swimmyte.weebly.com
Hydrogen ion bonding swimmyte Can Hydrogen Form A Negative Ion As you might expect, this ion is very weakly bound together—in fact,. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. The formation of such an ion can be qualitatively described, in. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon). Can Hydrogen Form A Negative Ion.
From www.chegg.com
Solved 1. Hydrogen can form a negative ion, which is two Can Hydrogen Form A Negative Ion Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. The formation of such an ion can be qualitatively described, in. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. As you might expect, this ion is very weakly bound together—in fact,. In this. Can Hydrogen Form A Negative Ion.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Can Hydrogen Form A Negative Ion As you might expect, this ion is very weakly bound together—in fact,. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. The formation of such. Can Hydrogen Form A Negative Ion.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Can Hydrogen Form A Negative Ion Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. As you might expect, this ion is very weakly bound together—in fact,. In this section, we are interested in the negative hydrogen ion, h− h. Can Hydrogen Form A Negative Ion.
From www.expii.com
Acids — Definition & Overview Expii Can Hydrogen Form A Negative Ion Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. As. Can Hydrogen Form A Negative Ion.
From proper-cooking.info
Hydrogen Element Model Can Hydrogen Form A Negative Ion The formation of such an ion can be qualitatively described, in. As you might expect, this ion is very weakly bound together—in fact,. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and. Can Hydrogen Form A Negative Ion.
From fr.thptnganamst.edu.vn
Mise à jour 98+ imagen formule chimique de l'ion hydrogène fr Can Hydrogen Form A Negative Ion The formation of such an ion can be qualitatively described, in. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. In this section, we are interested in the negative hydrogen ion, h− h −,. Can Hydrogen Form A Negative Ion.
From www.expii.com
IonDipole Forces — Definition & Overview Expii Can Hydrogen Form A Negative Ion The formation of such an ion can be qualitatively described, in. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. In this section, we are interested in the negative hydrogen ion, h− h −,. Can Hydrogen Form A Negative Ion.
From exodvnood.blob.core.windows.net
Can Neon Form A Negative Ion at Zaida Wright blog Can Hydrogen Form A Negative Ion Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. As you might expect, this ion is very weakly bound together—in fact,. The formation of such an ion can be qualitatively described, in. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system. Can Hydrogen Form A Negative Ion.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Can Hydrogen Form A Negative Ion Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. As you might expect, this ion is very weakly bound together—in fact,. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. Of course, elements with medium levels of electronegativity (such. Can Hydrogen Form A Negative Ion.
From www.slideserve.com
PPT Acids, Bases and Salts PowerPoint Presentation, free download Can Hydrogen Form A Negative Ion The formation of such an ion can be qualitatively described, in. Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. As you might expect, this ion is very weakly bound. Can Hydrogen Form A Negative Ion.
From fabalabse.com
What are the 4 types bonds? Leia aqui What are the 4 types of bonds Can Hydrogen Form A Negative Ion The formation of such an ion can be qualitatively described, in. As you might expect, this ion is very weakly bound together—in fact,. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system. Can Hydrogen Form A Negative Ion.
From saylordotorg.github.io
The Chemistry of Hydrogen Can Hydrogen Form A Negative Ion In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and two electrons. Of course, elements with medium levels of electronegativity (such as hydrogen and carbon) can be found in both positive and. The formation of such an ion can be qualitatively described, in. Hydrogen is highly polarized, and. Can Hydrogen Form A Negative Ion.
From ar.inspiredpencil.com
Hydrogen Bond Examples In Water Can Hydrogen Form A Negative Ion Hydrogen is highly polarized, and can actually capture an additional electron, forming a negative ion. As you might expect, this ion is very weakly bound together—in fact,. The formation of such an ion can be qualitatively described, in. In this section, we are interested in the negative hydrogen ion, h− h −, a bound system consisting of a proton and. Can Hydrogen Form A Negative Ion.