Catalysts Increase Reaction Rates By . Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst also increases the rate. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. A catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically unchanged at. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Heterogeneous catalysts, homogeneous catalysts, and enzymes. However, not all reactions have suitable. Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a substance that can. In this section, we will examine the three major classes of catalysts: See examples, diagrams and explanations of catalysis and collision theory. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. This page explains how adding a catalyst affects the rate of a reaction.
from www.numerade.com
A catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically unchanged at. This page explains how adding a catalyst affects the rate of a reaction. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. However, not all reactions have suitable. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Only a very small mass of catalyst is needed to increase the rate of a reaction. In this section, we will examine the three major classes of catalysts: Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. See examples, diagrams and explanations of catalysis and collision theory.
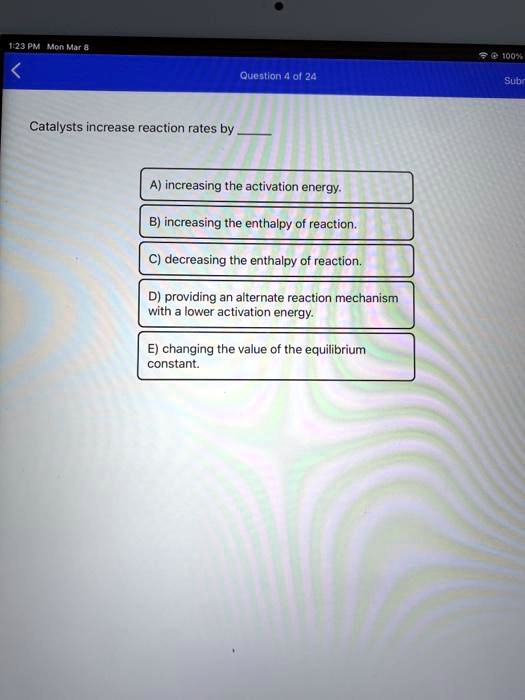
SOLVED Jr3Ar cneat Jout" Quostlon 0l 20 Subr Catalysts increase reaction rates by increasing
Catalysts Increase Reaction Rates By Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. Heterogeneous catalysts, homogeneous catalysts, and enzymes. This page explains how adding a catalyst affects the rate of a reaction. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. See examples, diagrams and explanations of catalysis and collision theory. A catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically unchanged at. Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a substance that can. Only a very small mass of catalyst is needed to increase the rate of a reaction. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. A catalyst also increases the rate. In this section, we will examine the three major classes of catalysts: However, not all reactions have suitable.
From www.slideserve.com
PPT Reaction Rates 2 PowerPoint Presentation, free download ID3493687 Catalysts Increase Reaction Rates By Heterogeneous catalysts, homogeneous catalysts, and enzymes. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst also increases the rate. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of. Catalysts Increase Reaction Rates By.
From fyoefmgmv.blob.core.windows.net
Do Catalysts Increase Reaction Rate at Stephen Johnson blog Catalysts Increase Reaction Rates By A catalyst also increases the rate. This page explains how adding a catalyst affects the rate of a reaction. Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a substance that can. A catalyst is a substance which increases the rate of a chemical reaction but it is not used up. Catalysts Increase Reaction Rates By.
From www.thesciencehive.co.uk
Reaction Rates* — the science sauce Catalysts Increase Reaction Rates By This page explains how adding a catalyst affects the rate of a reaction. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. A catalyst also increases the rate. Only a very small mass of. Catalysts Increase Reaction Rates By.
From byjus.com
How does a catalyst increase the rate of a reaction? Catalysts Increase Reaction Rates By Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a substance that can. Heterogeneous catalysts, homogeneous catalysts, and enzymes. A catalyst also increases the rate. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. In this section, we will examine the three major classes of catalysts:. Catalysts Increase Reaction Rates By.
From courses.lumenlearning.com
Factors Affecting Reaction Rates Chemistry Catalysts Increase Reaction Rates By This page explains how adding a catalyst affects the rate of a reaction. Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a substance that can. Heterogeneous catalysts, homogeneous catalysts, and enzymes. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. A catalyst is a substance. Catalysts Increase Reaction Rates By.
From study.com
Effect of Catalysts on Rates of Reaction Lesson Catalysts Increase Reaction Rates By A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. Among. Catalysts Increase Reaction Rates By.
From slideplayer.com
ENZYMES. ppt download Catalysts Increase Reaction Rates By Only a very small mass of catalyst is needed to increase the rate of a reaction. Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a substance that can. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. However, not all reactions have suitable. A. Catalysts Increase Reaction Rates By.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts Increase Reaction Rates By Only a very small mass of catalyst is needed to increase the rate of a reaction. See examples, diagrams and explanations of catalysis and collision theory. This page explains how adding a catalyst affects the rate of a reaction. Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a substance that. Catalysts Increase Reaction Rates By.
From slideplayer.com
Dr. Namphol Sinkaset Chem 201 General Chemistry II ppt download Catalysts Increase Reaction Rates By A catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically unchanged at. Heterogeneous catalysts, homogeneous catalysts, and enzymes. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. In this section, we will examine the three major classes of catalysts: Is a substance that increases. Catalysts Increase Reaction Rates By.
From www.numerade.com
SOLVED Enzymes are remarkably efficient catalysts that can increase reaction rates by as many Catalysts Increase Reaction Rates By A catalyst also increases the rate. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. In this section, we will examine the three major classes of catalysts:. Catalysts Increase Reaction Rates By.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalysts Increase Reaction Rates By Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a substance that can. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. Heterogeneous catalysts, homogeneous catalysts, and enzymes. In. Catalysts Increase Reaction Rates By.
From shapeguidance1.gitlab.io
Outrageous Does A Catalyst Increase The Rate Of Reaction Year 12 Physics Cheat Sheet Catalysts Increase Reaction Rates By Heterogeneous catalysts, homogeneous catalysts, and enzymes. This page explains how adding a catalyst affects the rate of a reaction. Only a very small mass of catalyst is needed to increase the rate of a reaction. See examples, diagrams and explanations of catalysis and collision theory. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction. Catalysts Increase Reaction Rates By.
From www.numerade.com
SOLVED Jr3Ar cneat Jout" Quostlon 0l 20 Subr Catalysts increase reaction rates by increasing Catalysts Increase Reaction Rates By Heterogeneous catalysts, homogeneous catalysts, and enzymes. A catalyst also increases the rate. This page explains how adding a catalyst affects the rate of a reaction. However, not all reactions have suitable. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. A catalyst is a substance which increases the rate of a chemical reaction but it. Catalysts Increase Reaction Rates By.
From slideplayer.com
Earth Chemistry Chapter ppt download Catalysts Increase Reaction Rates By See examples, diagrams and explanations of catalysis and collision theory. Only a very small mass of catalyst is needed to increase the rate of a reaction. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. Heterogeneous catalysts, homogeneous catalysts, and enzymes. This page explains how adding a catalyst affects the rate of a reaction. However,. Catalysts Increase Reaction Rates By.
From www.numerade.com
SOLVED Catalysts increase reaction rates by A) increasing the activation energy B) increasing Catalysts Increase Reaction Rates By However, not all reactions have suitable. A catalyst also increases the rate. See examples, diagrams and explanations of catalysis and collision theory. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. Heterogeneous catalysts, homogeneous catalysts, and enzymes. A. Catalysts Increase Reaction Rates By.
From slideplayer.com
Unit 8 and Rates of Reactions ppt download Catalysts Increase Reaction Rates By Heterogeneous catalysts, homogeneous catalysts, and enzymes. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. This page explains how adding a catalyst affects the rate of a reaction. A catalyst also increases the rate. However, not all reactions have suitable. Is a substance that increases the rate of reaction, but can be recovered, unchanged at. Catalysts Increase Reaction Rates By.
From slideplayer.com
Reaction Rates and Chemical Equilibrium ppt download Catalysts Increase Reaction Rates By This page explains how adding a catalyst affects the rate of a reaction. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. In this section, we will examine the three major classes of catalysts: A catalyst is. Catalysts Increase Reaction Rates By.
From sciencenotes.org
Factors That Affect Reaction Rate Chemical Catalysts Increase Reaction Rates By Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. A catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically unchanged at. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. However, not all reactions have. Catalysts Increase Reaction Rates By.
From www.slideserve.com
PPT Ch. 14Chemical PowerPoint Presentation, free download ID4450613 Catalysts Increase Reaction Rates By Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. Only a very small mass of catalyst is needed to increase the rate of a reaction. This page explains how adding a catalyst affects the rate of a reaction. See examples, diagrams and explanations of catalysis and collision theory. However, not all reactions. Catalysts Increase Reaction Rates By.
From slideplayer.com
Chemistry January 2 Reaction Rates. ppt download Catalysts Increase Reaction Rates By This page explains how adding a catalyst affects the rate of a reaction. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. A catalyst also increases the rate. In this section, we will examine the three major classes of catalysts: Heterogeneous catalysts, homogeneous catalysts, and enzymes. Among the factors affecting chemical reaction rates discussed earlier. Catalysts Increase Reaction Rates By.
From jackwestin.com
Rate Processes Catalysts Rate Processes In Chemical Reactions And Equilibrium MCAT Catalysts Increase Reaction Rates By Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a substance that can. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Only a very small mass of catalyst is needed to increase the rate of a reaction. This. Catalysts Increase Reaction Rates By.
From www.bartleby.com
Answered Catalysts increase reaction rates by A)… bartleby Catalysts Increase Reaction Rates By Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a substance that can. However, not all reactions have suitable. See examples, diagrams and explanations of catalysis and collision theory. In this section, we will examine the three major classes of catalysts: Because a catalyst decreases the height of the energy barrier,. Catalysts Increase Reaction Rates By.
From www.chegg.com
Solved Catalysts increase reaction rates by A) increasing Catalysts Increase Reaction Rates By However, not all reactions have suitable. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. See examples, diagrams and explanations of catalysis and collision theory. A catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically unchanged at.. Catalysts Increase Reaction Rates By.
From slideplayer.com
Chemical Reactions and Collision Theory ppt download Catalysts Increase Reaction Rates By Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence. Catalysts Increase Reaction Rates By.
From www.nagwa.com
Question Video Describing the Effect of Catalysts on Reaction Rates Nagwa Catalysts Increase Reaction Rates By However, not all reactions have suitable. Heterogeneous catalysts, homogeneous catalysts, and enzymes. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. Only a very small mass of. Catalysts Increase Reaction Rates By.
From slideplayer.com
Lecture 1405 Reaction Mechanism and Catalysis ppt download Catalysts Increase Reaction Rates By A catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically unchanged at. This page explains how adding a catalyst affects the rate of a reaction. In this section, we will examine the three major classes of catalysts: Only a very small mass of catalyst is needed to increase the. Catalysts Increase Reaction Rates By.
From slideplayer.com
Chemistry January 2 Reaction Rates. ppt download Catalysts Increase Reaction Rates By However, not all reactions have suitable. A catalyst also increases the rate. In this section, we will examine the three major classes of catalysts: Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. A catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically. Catalysts Increase Reaction Rates By.
From www.studypool.com
SOLUTION Rates of reaction catalysts mindmap Studypool Catalysts Increase Reaction Rates By A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. Is a substance that increases the rate of reaction, but can be recovered, unchanged at the end. Heterogeneous catalysts, homogeneous catalysts, and enzymes. In this section, we will examine the three major classes of catalysts: Learn how catalysts speed up reactions by providing an alternative route. Catalysts Increase Reaction Rates By.
From www.slideserve.com
PPT Factors Affecting Reaction Rates PowerPoint Presentation, free download ID3325825 Catalysts Increase Reaction Rates By However, not all reactions have suitable. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. Only a very small mass of catalyst is needed to increase the rate of a reaction. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst,. Catalysts Increase Reaction Rates By.
From www.slideserve.com
PPT Rates of Reactions PowerPoint Presentation, free download ID6770058 Catalysts Increase Reaction Rates By In this section, we will examine the three major classes of catalysts: A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. A catalyst is a substance which increases the rate of a chemical reaction but it is not used up. Catalysts Increase Reaction Rates By.
From www.slideserve.com
PPT Chemical Reactions and Collision Theory PowerPoint Presentation, free download ID1833784 Catalysts Increase Reaction Rates By A catalyst also increases the rate. Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. Heterogeneous catalysts, homogeneous catalysts, and enzymes. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Only a very small mass. Catalysts Increase Reaction Rates By.
From www.slideserve.com
PPT Ch. 14Chemical PowerPoint Presentation, free download ID4450613 Catalysts Increase Reaction Rates By Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. In this section, we will examine the three major classes of catalysts: Heterogeneous catalysts, homogeneous catalysts, and. Catalysts Increase Reaction Rates By.
From slideplayer.com
Controlling Reactions ppt download Catalysts Increase Reaction Rates By See examples, diagrams and explanations of catalysis and collision theory. A catalyst is a substance which increases the rate of a chemical reaction but it is not used up (remains chemically unchanged at. This page explains how adding a catalyst affects the rate of a reaction. Because a catalyst decreases the height of the energy barrier, its presence increases the. Catalysts Increase Reaction Rates By.
From www.numerade.com
SOLVED (a) Select all of the correct statements about reaction rates from the choices below Catalysts Increase Reaction Rates By Among the factors affecting chemical reaction rates discussed earlier in this chapter was the presence of a catalyst, a substance that can. A catalyst lowers the activation energy of a reaction by providing an alternative mechanism. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. However, not all reactions have suitable. See examples, diagrams. Catalysts Increase Reaction Rates By.
From slideplayer.com
7.3 Chapter ppt download Catalysts Increase Reaction Rates By Because a catalyst decreases the height of the energy barrier, its presence increases the reaction rates of both the forward and the reverse reactions by the same amount. A catalyst also increases the rate. Learn how catalysts speed up reactions by providing an alternative route with lower activation energy. Is a substance that increases the rate of reaction, but can. Catalysts Increase Reaction Rates By.