Chlorine Electrons To Fill Outer Shell . Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules,. A neutral chlorine atom has seven electrons in its outermost shell. Electrons in atoms occupy energy levels, also called electron shells, outside the. Different shells can hold different maximum numbers of. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. In contrast, chlorine has 2 electrons in the \(3s\) shell and 5 electrons in the \(3p\) shell. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. Only one more electron is needed to achieve an octet in. All it needs is one more electron to have. Each of the chlorine atoms gets an electron to fill its shell, and the aluminum loses three, giving it a filled shell too (remember, aluminum has three extra electrons). 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven electrons in its outermost.
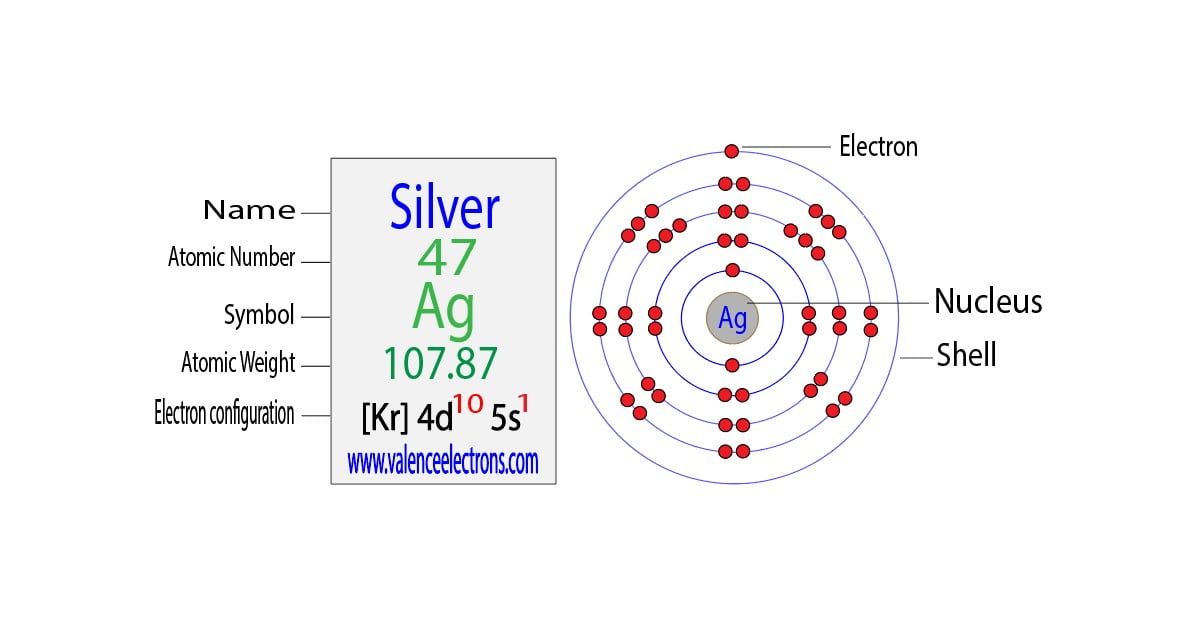
from valenceelectrons.com
Only one more electron is needed to achieve an octet in. Different shells can hold different maximum numbers of. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven electrons in its outermost. Each of the chlorine atoms gets an electron to fill its shell, and the aluminum loses three, giving it a filled shell too (remember, aluminum has three extra electrons). All it needs is one more electron to have. A neutral chlorine atom has seven electrons in its outermost shell. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules,. Electrons in atoms occupy energy levels, also called electron shells, outside the.
Electron Configuration for Chlorine (Cl) Full Explanation
Chlorine Electrons To Fill Outer Shell Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules,. In contrast, chlorine has 2 electrons in the \(3s\) shell and 5 electrons in the \(3p\) shell. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. A neutral chlorine atom has seven electrons in its outermost shell. 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven electrons in its outermost. Each of the chlorine atoms gets an electron to fill its shell, and the aluminum loses three, giving it a filled shell too (remember, aluminum has three extra electrons). Electrons in atoms occupy energy levels, also called electron shells, outside the. All it needs is one more electron to have. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. Only one more electron is needed to achieve an octet in. Different shells can hold different maximum numbers of. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules,.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Chlorine Electrons To Fill Outer Shell 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven electrons in its outermost. A neutral chlorine atom has seven electrons in its outermost shell. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. Each of the chlorine atoms gets. Chlorine Electrons To Fill Outer Shell.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry Chlorine Electrons To Fill Outer Shell 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven electrons in its outermost. Only one more electron is needed to achieve an octet in. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. In contrast, chlorine has 2 electrons in. Chlorine Electrons To Fill Outer Shell.
From www.britannica.com
Halogen Elements, Examples, Properties, Uses, & Facts Britannica Chlorine Electrons To Fill Outer Shell Electrons in atoms occupy energy levels, also called electron shells, outside the. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules,. In contrast, chlorine has 2 electrons in the \(3s\) shell and 5 electrons in the \(3p\) shell. All it. Chlorine Electrons To Fill Outer Shell.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Electrons To Fill Outer Shell In contrast, chlorine has 2 electrons in the \(3s\) shell and 5 electrons in the \(3p\) shell. Electrons in atoms occupy energy levels, also called electron shells, outside the. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. All it needs is one more electron to have. A neutral chlorine atom has. Chlorine Electrons To Fill Outer Shell.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Chlorine Electrons To Fill Outer Shell Only one more electron is needed to achieve an octet in. A neutral chlorine atom has seven electrons in its outermost shell. 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven electrons in its outermost. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost. Chlorine Electrons To Fill Outer Shell.
From philschatz.com
The Building Blocks of Molecules · Concepts of Biology Chlorine Electrons To Fill Outer Shell The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. A neutral chlorine atom has seven electrons in its outermost shell. 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven electrons in its outermost. Each of the chlorine atoms gets. Chlorine Electrons To Fill Outer Shell.
From ameblo.jp
Chlorine Electrons exarepec1973のブログ Chlorine Electrons To Fill Outer Shell A neutral chlorine atom has seven electrons in its outermost shell. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. Only one more electron is needed to achieve an octet in. Different shells can hold different maximum numbers of. Each of the chlorine atoms gets an electron to fill its shell,. Chlorine Electrons To Fill Outer Shell.
From www.nagwa.com
Question Video Identifying the Number of Electrons in the Outermost Electron Shell of an Atom Chlorine Electrons To Fill Outer Shell A neutral chlorine atom has seven electrons in its outermost shell. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. Only one more electron is needed to achieve an octet in. Different shells can hold different maximum numbers of. In contrast, chlorine has 2 electrons in the \(3s\) shell and 5 electrons. Chlorine Electrons To Fill Outer Shell.
From courses.lumenlearning.com
Reading Electrons Biology I Chlorine Electrons To Fill Outer Shell In contrast, chlorine has 2 electrons in the \(3s\) shell and 5 electrons in the \(3p\) shell. All it needs is one more electron to have. Electrons in atoms occupy energy levels, also called electron shells, outside the. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with. Chlorine Electrons To Fill Outer Shell.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Shutterstock Chlorine Electrons To Fill Outer Shell In contrast, chlorine has 2 electrons in the \(3s\) shell and 5 electrons in the \(3p\) shell. Electrons in atoms occupy energy levels, also called electron shells, outside the. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. 1 illustrates, a sodium atom (na) only has one electron in its outermost shell,. Chlorine Electrons To Fill Outer Shell.
From pixels.com
Chlorine Electron Configuration Photograph by Photo Libary Pixels Chlorine Electrons To Fill Outer Shell The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. Electrons in atoms occupy energy levels, also called electron shells, outside the. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules,. A. Chlorine Electrons To Fill Outer Shell.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science Chlorine Electrons To Fill Outer Shell Different shells can hold different maximum numbers of. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. Each of the chlorine atoms gets an electron to fill its shell, and the aluminum loses three, giving it a filled shell too (remember, aluminum has three extra electrons). Group 17 elements, including fluorine. Chlorine Electrons To Fill Outer Shell.
From www2.victoriacollege.edu
formation of ionic bonds Chlorine Electrons To Fill Outer Shell Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules,. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. In contrast, chlorine has 2 electrons in the \(3s\) shell and 5 electrons in. Chlorine Electrons To Fill Outer Shell.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Electrons To Fill Outer Shell 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven electrons in its outermost. Electrons in atoms occupy energy levels, also called electron shells, outside the. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an. Chlorine Electrons To Fill Outer Shell.
From valenceelectrons.com
How to Write the Electron Configuration for Chlorine (Cl)? Chlorine Electrons To Fill Outer Shell The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules,. Electrons in atoms occupy energy levels, also called electron shells, outside the. 1 illustrates,. Chlorine Electrons To Fill Outer Shell.
From slideplayer.com
Understanding Chemical Reactions Lesson Covalent bonding. ppt download Chlorine Electrons To Fill Outer Shell 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven electrons in its outermost. A neutral chlorine atom has seven electrons in its outermost shell. Only one more electron is needed to achieve an octet in. Electrons in atoms occupy energy levels, also called electron shells, outside the. Different. Chlorine Electrons To Fill Outer Shell.
From newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Chlorine Electrons To Fill Outer Shell 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven electrons in its outermost. A neutral chlorine atom has seven electrons in its outermost shell. In contrast, chlorine has 2 electrons in the \(3s\) shell and 5 electrons in the \(3p\) shell. The electron configurations of silicon (14 electrons),. Chlorine Electrons To Fill Outer Shell.
From animalia-life.club
Lewis Dot Structure For Chlorine Chlorine Electrons To Fill Outer Shell Only one more electron is needed to achieve an octet in. A neutral chlorine atom has seven electrons in its outermost shell. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has. Chlorine Electrons To Fill Outer Shell.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Electrons To Fill Outer Shell Only one more electron is needed to achieve an octet in. A neutral chlorine atom has seven electrons in its outermost shell. In contrast, chlorine has 2 electrons in the \(3s\) shell and 5 electrons in the \(3p\) shell. 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven. Chlorine Electrons To Fill Outer Shell.
From www.slideshare.net
Covalent Bond Chlorine Electrons To Fill Outer Shell 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven electrons in its outermost. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules,. All it needs is one more. Chlorine Electrons To Fill Outer Shell.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Chlorine Electrons To Fill Outer Shell 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven electrons in its outermost. A neutral chlorine atom has seven electrons in its outermost shell. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron. Chlorine Electrons To Fill Outer Shell.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Chlorine Electrons To Fill Outer Shell All it needs is one more electron to have. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven electrons in its outermost. Electrons in atoms occupy energy levels, also called. Chlorine Electrons To Fill Outer Shell.
From mammothmemory.net
A certain amount of electrons are allowed in certain shells Chlorine Electrons To Fill Outer Shell The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. In contrast, chlorine has 2 electrons in the \(3s\) shell and 5 electrons in the \(3p\) shell. All it needs is one more electron to. Chlorine Electrons To Fill Outer Shell.
From www.pinterest.com
chlorine Atom model project, Electron configuration, Atom model Chlorine Electrons To Fill Outer Shell Different shells can hold different maximum numbers of. All it needs is one more electron to have. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules,. 1 illustrates, a sodium atom (na) only has one electron in its outermost shell,. Chlorine Electrons To Fill Outer Shell.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Electrons To Fill Outer Shell Each of the chlorine atoms gets an electron to fill its shell, and the aluminum loses three, giving it a filled shell too (remember, aluminum has three extra electrons). The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. Different shells can hold different maximum numbers of. Group 17 elements, including fluorine. Chlorine Electrons To Fill Outer Shell.
From slideplayer.com
BioChemistry. ppt download Chlorine Electrons To Fill Outer Shell 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven electrons in its outermost. Only one more electron is needed to achieve an octet in. Different shells can hold different maximum numbers of. A neutral chlorine atom has seven electrons in its outermost shell. All it needs is one. Chlorine Electrons To Fill Outer Shell.
From www.slideserve.com
PPT Introduction to Ionic Compounds PowerPoint Presentation ID690533 Chlorine Electrons To Fill Outer Shell The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven electrons in its outermost. All it needs is one more electron to have. The electron configurations of silicon (14 electrons), phosphorus. Chlorine Electrons To Fill Outer Shell.
From utedzz.blogspot.com
Periodic Table Chlorine Bohr Model Periodic Table Timeline Chlorine Electrons To Fill Outer Shell Only one more electron is needed to achieve an octet in. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules,. In contrast,. Chlorine Electrons To Fill Outer Shell.
From sciencenotes.org
Chlorine Facts Chlorine Electrons To Fill Outer Shell Different shells can hold different maximum numbers of. 1 illustrates, a sodium atom (na) only has one electron in its outermost shell, whereas a chlorine atom (cl) has seven electrons in its outermost. Each of the chlorine atoms gets an electron to fill its shell, and the aluminum loses three, giving it a filled shell too (remember, aluminum has three. Chlorine Electrons To Fill Outer Shell.
From chemtech-us.com
15 Interesting Facts About Chlorine Chlorine Electrons To Fill Outer Shell In contrast, chlorine has 2 electrons in the \(3s\) shell and 5 electrons in the \(3p\) shell. Each of the chlorine atoms gets an electron to fill its shell, and the aluminum loses three, giving it a filled shell too (remember, aluminum has three extra electrons). The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine. Chlorine Electrons To Fill Outer Shell.
From www.scienceabc.com
What Are Valence Electrons And How To Find Them? Where Are They Located? Chlorine Electrons To Fill Outer Shell The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules,. A neutral chlorine atom has seven electrons in its outermost shell. In contrast,. Chlorine Electrons To Fill Outer Shell.
From egpat.com
Lewis dot structure How to write? Chlorine Electrons To Fill Outer Shell Different shells can hold different maximum numbers of. All it needs is one more electron to have. A neutral chlorine atom has seven electrons in its outermost shell. Only one more electron is needed to achieve an octet in. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and. 1 illustrates, a sodium. Chlorine Electrons To Fill Outer Shell.
From valenceelectrons.com
Electron Configuration for Chlorine (Cl) Full Explanation Chlorine Electrons To Fill Outer Shell In contrast, chlorine has 2 electrons in the \(3s\) shell and 5 electrons in the \(3p\) shell. The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. Different shells can hold different maximum numbers of. All it needs is one more electron to have. A neutral chlorine atom has seven electrons in. Chlorine Electrons To Fill Outer Shell.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Chlorine Electrons To Fill Outer Shell A neutral chlorine atom has seven electrons in its outermost shell. All it needs is one more electron to have. Each of the chlorine atoms gets an electron to fill its shell, and the aluminum loses three, giving it a filled shell too (remember, aluminum has three extra electrons). Group 17 elements, including fluorine and chlorine, have seven electrons in. Chlorine Electrons To Fill Outer Shell.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together? Owlcation Chlorine Electrons To Fill Outer Shell The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon. All it needs is one more electron to have. Group 17 elements, including fluorine and chlorine, have seven electrons in their outmost shells, so they tend to fill this shell with an electron from other atoms or molecules,. Different shells can hold. Chlorine Electrons To Fill Outer Shell.