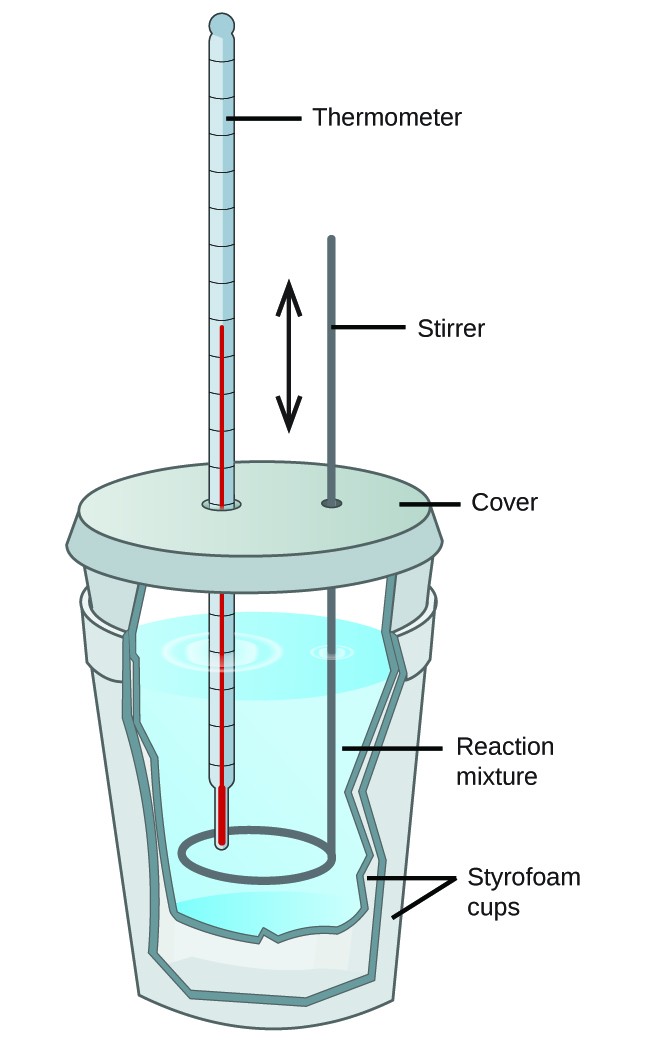
Calorimetry Heat Definition . a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat involved in a chemical or physical. measuring heat flow. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction.
from courses.lumenlearning.com
in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat involved in a chemical or physical. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. For example, when an exothermic reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. measuring heat flow.
Calorimetry Chemistry for Majors
Calorimetry Heat Definition One technique we can use to measure the amount of heat involved in a chemical or physical. measuring heat flow. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat involved in a chemical or physical. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic reaction. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
From slidetodoc.com
CALORIMETRY ENTHALPY CHANGES be able to define enthalpy Calorimetry Heat Definition One technique we can use to measure the amount of heat involved in a chemical or physical. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. For example, when an exothermic reaction. calorimetry is a field of thermochemistry that. Calorimetry Heat Definition.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types, Examples and Uses CollegeSearch Calorimetry Heat Definition One technique we can use to measure the amount of heat involved in a chemical or physical. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. a container that prevents heat transfer in. Calorimetry Heat Definition.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation & Application Calorimetry Heat Definition a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat. Calorimetry Heat Definition.
From slidetodoc.com
CALORIMETRY ENTHALPY CHANGES be able to define enthalpy Calorimetry Heat Definition calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter. Calorimetry Heat Definition.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Heat Definition a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat. Calorimetry Heat Definition.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Heat Definition calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. One technique we can use to measure the amount of heat involved in a chemical or physical. a calorimeter is a. Calorimetry Heat Definition.
From studylib.net
Calorimetry Calorimetry Heat Definition a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. One technique we can use to measure the amount of heat involved in a chemical or physical. For example, when an exothermic reaction. a calorimeter is a device used to. Calorimetry Heat Definition.
From learningcampusscarf.z13.web.core.windows.net
How To Calculate Heat Of Reaction Calorimetry Calorimetry Heat Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. measuring heat flow. a container that prevents heat transfer in or out is called a calorimeter, and the. Calorimetry Heat Definition.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Calorimetry Heat Definition For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the amount of heat involved. Calorimetry Heat Definition.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow (M6Q5) UWMadison Calorimetry Heat Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. One technique we can use to measure the amount of heat involved in a chemical or. Calorimetry Heat Definition.
From scienceinfo.com
Calorimetry Definition, Principle, Types, Application, and Limitations Calorimetry Heat Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Calorimetry Heat Definition.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Heat Definition For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. measuring heat flow. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the. Calorimetry Heat Definition.
From users.highland.edu
Calorimetry Calorimetry Heat Definition One technique we can use to measure the amount of heat involved in a chemical or physical. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. a calorimeter is a device used to measure the amount of heat involved. Calorimetry Heat Definition.
From slidetodoc.com
CALORIMETRY ENTHALPY CHANGES be able to define enthalpy Calorimetry Heat Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter. Calorimetry Heat Definition.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter Calorimetry Heat Definition a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount. Calorimetry Heat Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Heat Definition One technique we can use to measure the amount of heat involved in a chemical or physical. measuring heat flow. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical. Calorimetry Heat Definition.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Heat Definition measuring heat flow. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a field of thermochemistry that measures the amount of heat involved in a. Calorimetry Heat Definition.
From slidetodoc.com
CALORIMETRY ENTHALPY CHANGES be able to define enthalpy Calorimetry Heat Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat involved in a chemical or physical. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. a calorimeter is a device. Calorimetry Heat Definition.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tecscience Calorimetry Heat Definition One technique we can use to measure the amount of heat involved in a chemical or physical. measuring heat flow. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. a calorimeter is a device used to measure the. Calorimetry Heat Definition.
From slidetodoc.com
CALORIMETRY ENTHALPY CHANGES be able to define enthalpy Calorimetry Heat Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. measuring. Calorimetry Heat Definition.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Calorimetry Heat Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. calorimetry is. Calorimetry Heat Definition.
From slidetodoc.com
CALORIMETRY ENTHALPY CHANGES be able to define enthalpy Calorimetry Heat Definition measuring heat flow. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. . Calorimetry Heat Definition.
From slidetodoc.com
CALORIMETRY ENTHALPY CHANGES be able to define enthalpy Calorimetry Heat Definition in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. One technique we can use to measure the amount of heat involved in a chemical or physical. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device. Calorimetry Heat Definition.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry Calorimetry Heat Definition in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. One technique we can use to measure the amount of heat involved in a chemical or physical. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to. Calorimetry Heat Definition.
From slidetodoc.com
CALORIMETRY ENTHALPY CHANGES be able to define enthalpy Calorimetry Heat Definition measuring heat flow. One technique we can use to measure the amount of heat involved in a chemical or physical. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Calorimetry Heat Definition.
From exoarnnpu.blob.core.windows.net
Calorimeter Description And Function at Kyla Ochs blog Calorimetry Heat Definition a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. measuring heat flow. For example, when an exothermic reaction. One technique we can use to measure the amount of heat involved in a chemical or physical. a calorimeter is. Calorimetry Heat Definition.
From study.com
Calorimetry Definition, Equation & Types Lesson Calorimetry Heat Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Calorimetry Heat Definition.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Heat Definition in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. a calorimeter is a device used to measure the amount of heat involved. Calorimetry Heat Definition.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Heat Definition calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. For example, when an exothermic reaction. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical. Calorimetry Heat Definition.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimetry Heat Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved. Calorimetry Heat Definition.
From slidetodoc.com
CALORIMETRY ENTHALPY CHANGES be able to define enthalpy Calorimetry Heat Definition calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. measuring heat flow. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the. Calorimetry Heat Definition.
From scienceinfo.com
Calorimeter Definition, Types and Uses Calorimetry Heat Definition For example, when an exothermic reaction. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical. Calorimetry Heat Definition.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Calorimetry Heat Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. measuring heat flow. One technique we can use to measure the amount of heat involved in a chemical or. Calorimetry Heat Definition.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimetry Heat Definition a container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. a calorimeter is a device used to measure the amount of heat involved. Calorimetry Heat Definition.
From slidetodoc.com
CALORIMETRY ENTHALPY CHANGES be able to define enthalpy Calorimetry Heat Definition For example, when an exothermic reaction. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. measuring heat flow. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat involved. Calorimetry Heat Definition.