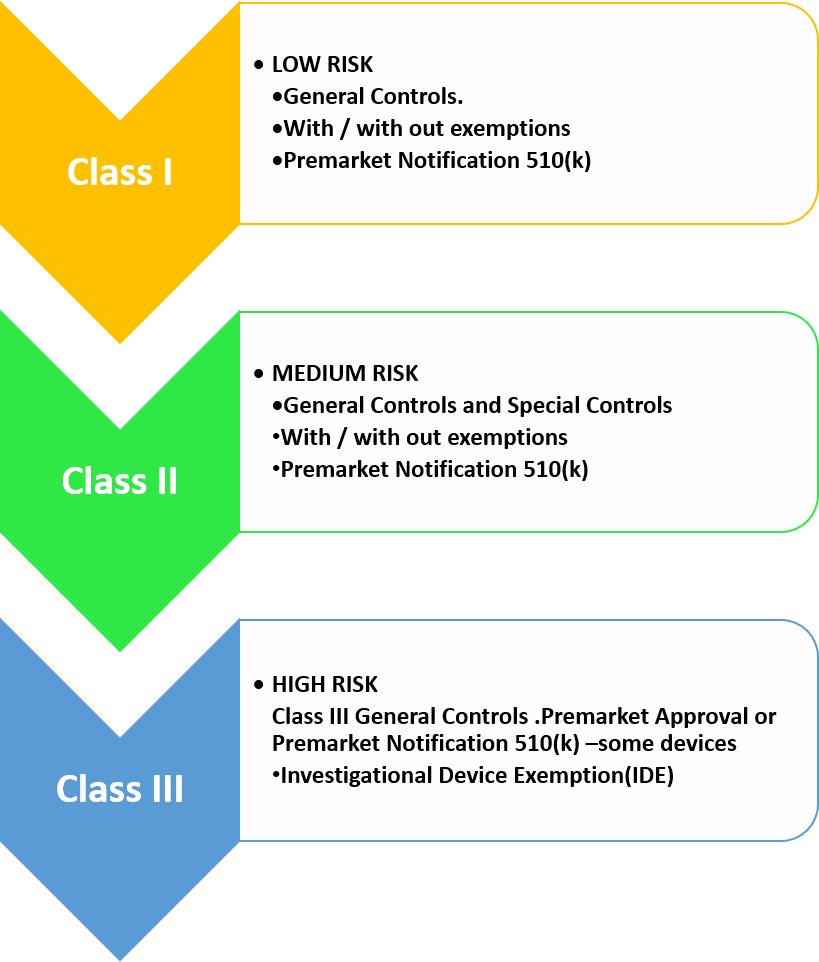
Medical Devices And Fda . Navigate the medical device section. The following information is available: Quick & easy compliancelow flat fee Welcome to fda's information about medical device approvals. Comprehensive list of latest cdrh updates. Medical devices range from something as simple as an elastic bandage to something as complicated as a. Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for use in. Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have included special pathways to accelerate availability of Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated.
from meddev-info.blogspot.com
The following information is available: Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for use in. Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have included special pathways to accelerate availability of Welcome to fda's information about medical device approvals. Medical devices range from something as simple as an elastic bandage to something as complicated as a. Comprehensive list of latest cdrh updates. Navigate the medical device section. Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. Quick & easy compliancelow flat fee
Medical Device Regulation Basics US FDA Medical Device Classification
Medical Devices And Fda Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. Quick & easy compliancelow flat fee Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have included special pathways to accelerate availability of Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. Medical devices range from something as simple as an elastic bandage to something as complicated as a. Comprehensive list of latest cdrh updates. Navigate the medical device section. Welcome to fda's information about medical device approvals. The following information is available: Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for use in.
From aaos.org
An Overview of the FDA Approval Process for Devices Medical Devices And Fda Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have included special pathways to accelerate availability of Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for use in.. Medical Devices And Fda.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations MedicalDevices FDA Medical Devices And Fda Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have included special pathways to accelerate availability of Navigate the medical device section. Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can. Medical Devices And Fda.
From medium.com
The 3 FDA medical device classes [differences and examples explained] by Qualio Medium Medical Devices And Fda Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. Medical devices range from something as simple as an elastic bandage to something as complicated as a. The following information is available: Quick & easy compliancelow flat fee Navigate the medical device section. Welcome to fda's information about medical device approvals. Similar. Medical Devices And Fda.
From phs.weill.cornell.edu
Food and Drug Administration Surveillance and Medical Device Regulation Population Health Sciences Medical Devices And Fda Welcome to fda's information about medical device approvals. Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have included special pathways to accelerate availability of Quick & easy compliancelow flat fee Comprehensive list of latest cdrh updates. Navigate the medical device section. Medical devices range from something. Medical Devices And Fda.
From fr.slideshare.net
Medical Device FDA Regulations and Classifications infographic Medical Devices And Fda Comprehensive list of latest cdrh updates. Medical devices range from something as simple as an elastic bandage to something as complicated as a. The following information is available: Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for use in. Fda has. Medical Devices And Fda.
From www.regdesk.co
FDA Guidance on eCopy Program for Medical Device Submissions RegDesk Medical Devices And Fda Medical devices range from something as simple as an elastic bandage to something as complicated as a. Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have included special. Medical Devices And Fda.
From www.pinterest.com
Infographic on Understanding FDA Device Classes from the leading EMS medical Medical Devices And Fda Comprehensive list of latest cdrh updates. Navigate the medical device section. Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have included special pathways to accelerate availability of Medical. Medical Devices And Fda.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical devices YouTube Medical Devices And Fda Welcome to fda's information about medical device approvals. Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have included special pathways to accelerate availability of Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. The following information is. Medical Devices And Fda.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Medical Devices And Fda Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. The following information is available: Welcome to fda's information about medical device approvals. Medical devices range from something as simple as an elastic bandage to something as complicated as a. Navigate the medical device section. Comprehensive list of latest cdrh updates. Similar. Medical Devices And Fda.
From gxp-training.com
GMP for Medical Devices Online Training FDA 21 CFR PART 820 Medical Devices And Fda Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. Quick & easy compliancelow flat fee Comprehensive list of latest cdrh updates. Welcome to fda's information about medical device approvals. The following information is available: Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations. Medical Devices And Fda.
From www.greenlight.guru
FDA Cleared vs Approved vs Granted for Medical Devices Medical Devices And Fda Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have included special pathways to accelerate availability of Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. The following information is available: Navigate the medical device section. Comprehensive list. Medical Devices And Fda.
From www.vrogue.co
The 3 Fda Medical Device Classes Differences And Exam vrogue.co Medical Devices And Fda Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have included special pathways to accelerate availability of Welcome to fda's information about medical device approvals. The following information is available: Navigate the medical device section. Comprehensive list of latest cdrh updates. Similar to drugs, medical devices in. Medical Devices And Fda.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Devices And Fda Navigate the medical device section. Comprehensive list of latest cdrh updates. Welcome to fda's information about medical device approvals. Quick & easy compliancelow flat fee The following information is available: Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. Similar to drugs, medical devices in the united states go through a. Medical Devices And Fda.
From www.presentationeze.com
FDA medical device classification PresentationEZE Medical Devices And Fda The following information is available: Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. Quick & easy compliancelow flat fee Comprehensive list of latest cdrh updates. Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can. Medical Devices And Fda.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Devices And Fda Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for use in. Medical devices range from something as simple as an elastic bandage to something as complicated as a. Fda has expectations for different aspects of digital health technologies, including when used. Medical Devices And Fda.
From sterlingmedicaldevices.com
FDA Medical Device Regulation Guidance for 2022 Medical Devices And Fda Medical devices range from something as simple as an elastic bandage to something as complicated as a. Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for use in. Fda has expectations for different aspects of digital health technologies, including when used. Medical Devices And Fda.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Devices And Fda Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for use in. The following information is available: Welcome to fda's information about medical device approvals. Navigate the medical device section. Medical devices range from something as simple as an elastic bandage to. Medical Devices And Fda.
From medtechintelligence.com
Column Compliance Date Approaching for FDA Unique Device Identifiers MedTech Intelligence Medical Devices And Fda Welcome to fda's information about medical device approvals. Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have included special pathways to accelerate availability of Comprehensive list of latest cdrh updates. The following information is available: Fda has expectations for different aspects of digital health technologies, including. Medical Devices And Fda.
From ramtechno.com
What Are the Three FDA Classes for Medical Devices? RAM Technologies Medical Devices And Fda Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for use in. Welcome to fda's information about medical device approvals. Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. Medical devices range from. Medical Devices And Fda.
From www.greenlight.guru
Understanding the FDA Medical Device Classification System Medical Devices And Fda Welcome to fda's information about medical device approvals. The following information is available: Medical devices range from something as simple as an elastic bandage to something as complicated as a. Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. Navigate the medical device section. Quick & easy compliancelow flat fee Comprehensive. Medical Devices And Fda.
From ramtechno.com
AI Medical Devices and FDA Approval A Review in The Lancet RAM Technologies Medical Devices And Fda Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have included special pathways to accelerate availability of Quick & easy compliancelow flat fee Comprehensive list of latest cdrh updates. Similar to drugs, medical devices in the united states go through a review process by the us food. Medical Devices And Fda.
From www.rimsys.io
FDA listed, cleared, approved, granted what do these mean, and what’s the difference? Medical Devices And Fda Navigate the medical device section. Welcome to fda's information about medical device approvals. Medical devices range from something as simple as an elastic bandage to something as complicated as a. The following information is available: Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can. Medical Devices And Fda.
From angelanjohnson.com
DrugDevice Combination Treatments and Diagnostics Roundtable Angela N Johnson Medical Devices And Fda Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for use in. Navigate the medical device section. The following information is available: Welcome to fda's information about medical device approvals. Comprehensive list of latest cdrh updates. Quick & easy compliancelow flat fee. Medical Devices And Fda.
From www.swisstechnologiesne.com
Medical Devices and The FDA Medical Devices And Fda Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for use in. Welcome to fda's information about medical device approvals. Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system. Medical Devices And Fda.
From medicalfuturist.com
FDAApproved AIBased Medical Devices The Medical Futurist Medical Devices And Fda Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have included special pathways to accelerate availability of Navigate the medical device section. The following information is available: Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. Welcome to. Medical Devices And Fda.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Devices And Fda Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. The following information is available: Comprehensive list of latest cdrh updates. Medical devices range from something as simple as an elastic bandage to something as complicated as a. Welcome to fda's information about medical device approvals. Findings since congress and the fda. Medical Devices And Fda.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Medical Devices And Fda The following information is available: Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. Quick & easy compliancelow flat fee Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for use in. Comprehensive. Medical Devices And Fda.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Devices And Fda Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for use in. Welcome to fda's information about medical device approvals. Quick & easy compliancelow flat fee The following information is available: Fda has expectations for different aspects of digital health technologies, including. Medical Devices And Fda.
From healthtrustpg.com
FDA Approval Update HealthTrust Performance Improvement For Healthcare Medical Devices And Fda Navigate the medical device section. Welcome to fda's information about medical device approvals. Quick & easy compliancelow flat fee Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have included special pathways to accelerate availability of Fda has expectations for different aspects of digital health technologies, including. Medical Devices And Fda.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Medical Devices And Fda The following information is available: Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for use in. Quick & easy compliancelow flat fee Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. Welcome. Medical Devices And Fda.
From www.rarediseaseadvisor.com
RealWorld Data, Evidence Help Drive Rare Disease Drug and Device Approval Medical Devices And Fda Comprehensive list of latest cdrh updates. Welcome to fda's information about medical device approvals. Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. Navigate the medical device section. Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have. Medical Devices And Fda.
From www.qualio.com
The 3 FDA medical device classes differences and examples explained Medical Devices And Fda Comprehensive list of latest cdrh updates. Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental innovations in the device regulation system have included special pathways to accelerate availability of Welcome to fda's information about medical device approvals. Quick & easy compliancelow flat fee Fda has expectations for different aspects of digital health technologies,. Medical Devices And Fda.
From www.regdesk.co
FDA Guidance on Testing and Labelling Medical Devices for Safety in the Resonance Medical Devices And Fda Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for use in. Quick & easy compliancelow flat fee Welcome to fda's information about medical device approvals. Findings since congress and the fda initiated premarket review of medical devices in 1976, some fundamental. Medical Devices And Fda.
From www.medtechimpact.com
FDA Focuses on Medical Device Safety • Medtech Impact On Wellness Medical Devices And Fda Navigate the medical device section. Fda has expectations for different aspects of digital health technologies, including when used as a medical product, incorporated. The following information is available: Welcome to fda's information about medical device approvals. Quick & easy compliancelow flat fee Medical devices range from something as simple as an elastic bandage to something as complicated as a. Findings. Medical Devices And Fda.
From www.regdesk.co
FDA on Reusable Medical Devices and Reprocessing RegDesk Medical Devices And Fda Similar to drugs, medical devices in the united states go through a review process by the us food and drug administration (fda) before they can be marketed for use in. Navigate the medical device section. Comprehensive list of latest cdrh updates. Welcome to fda's information about medical device approvals. Quick & easy compliancelow flat fee The following information is available:. Medical Devices And Fda.