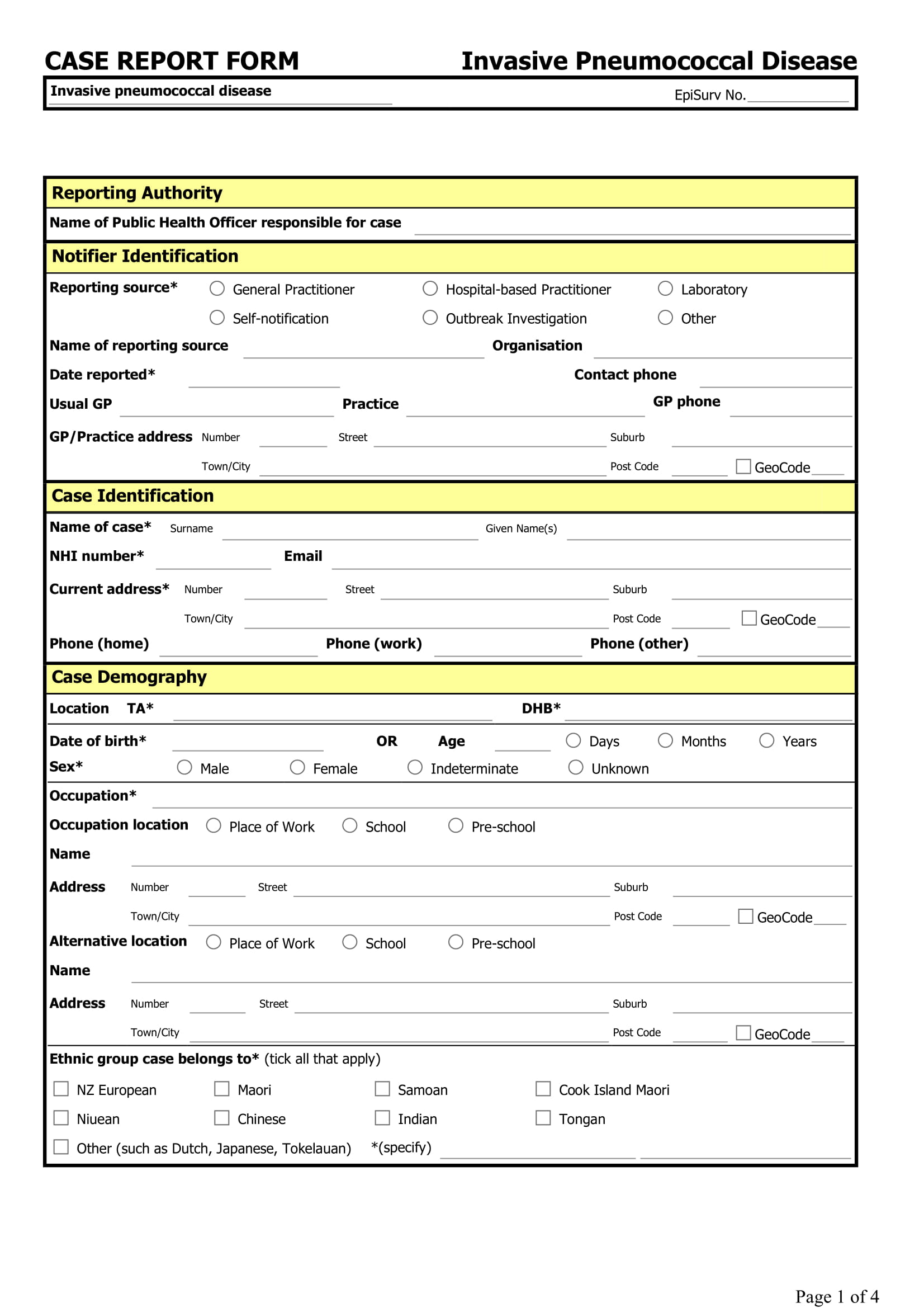
Case Report Form Design . Key design elements and inclusion of standard. Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: The purpose of this sop is to describe the procedure to be followed when designing, using and. This document sets out the procedures to be followed by all staff for designing a case report form (crf) either in paper (pcrf) or electronic. 1 introduction, background and purpose. Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. The purpose of this standard operating procedure (sop) is to describe the standard procedures to be followed when. The necessity of incorporating data sharing requirements. It may be a paper. A case report form (crf), is a printed, optical or electronic document designed to record all of the protocol required information to be reported to the sponsor on each trial subject,. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject.
from www.sampleforms.com
Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: This document sets out the procedures to be followed by all staff for designing a case report form (crf) either in paper (pcrf) or electronic. 1 introduction, background and purpose. Key design elements and inclusion of standard. It may be a paper. The necessity of incorporating data sharing requirements. A case report form (crf), is a printed, optical or electronic document designed to record all of the protocol required information to be reported to the sponsor on each trial subject,. The purpose of this sop is to describe the procedure to be followed when designing, using and.
FREE 15+ Case Report Forms in PDF MS Word
Case Report Form Design Key design elements and inclusion of standard. Key design elements and inclusion of standard. Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. A case report form (crf), is a printed, optical or electronic document designed to record all of the protocol required information to be reported to the sponsor on each trial subject,. The necessity of incorporating data sharing requirements. This document sets out the procedures to be followed by all staff for designing a case report form (crf) either in paper (pcrf) or electronic. Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: The purpose of this standard operating procedure (sop) is to describe the standard procedures to be followed when. 1 introduction, background and purpose. It may be a paper. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. The purpose of this sop is to describe the procedure to be followed when designing, using and.
From steps.kontenterkini.com
Case Report Form Template Sample Design Templates Case Report Form Design A case report form (crf), is a printed, optical or electronic document designed to record all of the protocol required information to be reported to the sponsor on each trial subject,. Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: 1 introduction, background and purpose. 1.2 the. Case Report Form Design.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Design Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. The purpose of this sop is to describe the procedure to be followed when designing, using and. Key design elements. Case Report Form Design.
From www.greenlight.guru
How to Design an Electronic Case Report Form (eCRF) for Medical Device Case Report Form Design Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: A case report form (crf), is a printed, optical or electronic document designed to record all of the protocol required information to be reported to the sponsor on each trial subject,. 1 introduction, background and purpose. Definitions case. Case Report Form Design.
From templatedbc.web.app
Crf Case Report Form Templates PrintableDB.web.app Case Report Form Design 1 introduction, background and purpose. The necessity of incorporating data sharing requirements. Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject.. Case Report Form Design.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Design It may be a paper. The necessity of incorporating data sharing requirements. The purpose of this sop is to describe the procedure to be followed when designing, using and. 1 introduction, background and purpose. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject.. Case Report Form Design.
From www.template.net
Case Report Form Template in Word, Pages, Google Docs Download Case Report Form Design The necessity of incorporating data sharing requirements. Key design elements and inclusion of standard. A case report form (crf), is a printed, optical or electronic document designed to record all of the protocol required information to be reported to the sponsor on each trial subject,. The purpose of this standard operating procedure (sop) is to describe the standard procedures to. Case Report Form Design.
From www.swiftsparks.com
Case Report Form Template PROFESSIONAL TEMPLATES PROFESSIONAL TEMPLATES Case Report Form Design 1 introduction, background and purpose. Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. This document sets out the procedures to be followed by all staff for designing a case report form (crf) either in paper (pcrf) or electronic. The purpose of this sop is to describe the procedure to be. Case Report Form Design.
From besttemplate-ideas.blogspot.com
Case Report Form Template Best Template Ideas Case Report Form Design A case report form (crf), is a printed, optical or electronic document designed to record all of the protocol required information to be reported to the sponsor on each trial subject,. 1 introduction, background and purpose. Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: 1.2 the. Case Report Form Design.
From www.slideserve.com
PPT Case Report Form Design Module Three Best Practices PowerPoint Case Report Form Design Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. The purpose of this standard operating procedure (sop) is to describe the standard procedures to be followed when. 1 introduction,. Case Report Form Design.
From www.sampletemplates.com
FREE 12+ Sample Case Report Templates in PDF MS Word Google Docs Case Report Form Design The purpose of this sop is to describe the procedure to be followed when designing, using and. The necessity of incorporating data sharing requirements. The purpose of this standard operating procedure (sop) is to describe the standard procedures to be followed when. Key design elements and inclusion of standard. Depending on the data required by the protocol, a standard crf. Case Report Form Design.
From www.sampletemplate.my.id
Case Report Form Template Sampletemplate.my.id Case Report Form Design 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. The necessity of incorporating data sharing requirements. 1 introduction, background and purpose. It may be a. Case Report Form Design.
From www.pdffiller.com
Fillable Online Fillable Online Design of case report forms based on a Case Report Form Design Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. This document sets out the procedures to be followed by all staff for designing a case report form (crf) either in paper (pcrf) or electronic. Depending on the data required by the protocol, a standard crf document might include, but is not. Case Report Form Design.
From www.sampletemplate.my.id
Case Report Form Template Sampletemplate.my.id Case Report Form Design Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: The purpose of this sop is to describe the procedure to be followed when designing, using and. It may be a paper. 1.2 the case report form (crf) is a data collection tool used to capture the required. Case Report Form Design.
From www.slideserve.com
PPT Design of Case Report Forms PowerPoint Presentation ID226993 Case Report Form Design It may be a paper. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Key design elements and inclusion of standard. The purpose of this standard operating procedure (sop) is to describe the standard procedures to be followed when. This document sets out. Case Report Form Design.
From business.fromgrandma.best
Design Of Case Report Forms Based On A Public Metadata In Case Report Case Report Form Design Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. It may be a paper. A case report form (crf), is a printed, optical or electronic document designed to record. Case Report Form Design.
From www.researchgate.net
(PDF) Basics of case report form designing in clinical research Case Report Form Design This document sets out the procedures to be followed by all staff for designing a case report form (crf) either in paper (pcrf) or electronic. The necessity of incorporating data sharing requirements. The purpose of this standard operating procedure (sop) is to describe the standard procedures to be followed when. Depending on the data required by the protocol, a standard. Case Report Form Design.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Design A case report form (crf), is a printed, optical or electronic document designed to record all of the protocol required information to be reported to the sponsor on each trial subject,. Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: 1 introduction, background and purpose. Key design. Case Report Form Design.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Design This document sets out the procedures to be followed by all staff for designing a case report form (crf) either in paper (pcrf) or electronic. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. 1 introduction, background and purpose. Definitions case report form. Case Report Form Design.
From www.template.net
Case Report Form Template in Word, Pages, Google Docs Download Case Report Form Design The purpose of this sop is to describe the procedure to be followed when designing, using and. Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: A case report form (crf), is a printed, optical or electronic document designed to record all of the protocol required information. Case Report Form Design.
From www.sampletemplate.my.id
Case Report Form Template Sampletemplate.my.id Case Report Form Design 1 introduction, background and purpose. This document sets out the procedures to be followed by all staff for designing a case report form (crf) either in paper (pcrf) or electronic. Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: The purpose of this standard operating procedure (sop). Case Report Form Design.
From cloudflare.itsnudimension.com
Case Report Form Template Creative Sample Templates Case Report Form Design Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: It may be a paper. The necessity of incorporating data sharing requirements. This document sets out the procedures to be followed by all staff for designing a case report form (crf) either in paper (pcrf) or electronic. Definitions. Case Report Form Design.
From paradigmit.com
Electronic Case Report Form (eCRF) Design ParadigmIT Case Report Form Design The purpose of this sop is to describe the procedure to be followed when designing, using and. Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. The necessity of incorporating data sharing requirements. Key design elements and inclusion of standard. It may be a paper. 1.2 the case report form (crf). Case Report Form Design.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Design 1 introduction, background and purpose. This document sets out the procedures to be followed by all staff for designing a case report form (crf) either in paper (pcrf) or electronic. Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: 1.2 the case report form (crf) is a. Case Report Form Design.
From www.pdffiller.com
Fillable Online icssc Case Report Form Design Research Informatics Case Report Form Design Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. This document sets out the procedures to be followed by all staff for designing a case. Case Report Form Design.
From www.template.net
Case Report Form Template in Word, Pages, Google Docs Download Case Report Form Design The purpose of this sop is to describe the procedure to be followed when designing, using and. A case report form (crf), is a printed, optical or electronic document designed to record all of the protocol required information to be reported to the sponsor on each trial subject,. It may be a paper. The necessity of incorporating data sharing requirements.. Case Report Form Design.
From clickup.com
15 Best Case Study Templates to Use in Word and ClickUp Case Report Form Design A case report form (crf), is a printed, optical or electronic document designed to record all of the protocol required information to be reported to the sponsor on each trial subject,. Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. The purpose of this sop is to describe the procedure to. Case Report Form Design.
From www.scientific.net
Based Case Report Form Design for Clinical Trial Case Report Form Design Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. The purpose of this sop is to describe the procedure to be followed when designing, using and. Key design elements and inclusion of standard. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as. Case Report Form Design.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Design A case report form (crf), is a printed, optical or electronic document designed to record all of the protocol required information to be reported to the sponsor on each trial subject,. Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. The necessity of incorporating data sharing requirements. Depending on the data. Case Report Form Design.
From www.smart-trial.com
How to Design an Electronic Case Report Form (eCRF) Case Report Form Design 1 introduction, background and purpose. 1.2 the case report form (crf) is a data collection tool used to capture the required data, as defined by the protocol, for each individual subject. Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: Definitions case report form (crf) form on. Case Report Form Design.
From www.slideserve.com
PPT Design of Case Report Forms PowerPoint Presentation, free Case Report Form Design The purpose of this standard operating procedure (sop) is to describe the standard procedures to be followed when. Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: This document sets out the procedures to be followed by all staff for designing a case report form (crf) either. Case Report Form Design.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Case Report Form Design 1 introduction, background and purpose. Depending on the data required by the protocol, a standard crf document might include, but is not limited to, the following pages: The purpose of this standard operating procedure (sop) is to describe the standard procedures to be followed when. This document sets out the procedures to be followed by all staff for designing a. Case Report Form Design.
From www.template.net
Case Report Form Template in Word, Pages, Google Docs Download Case Report Form Design Key design elements and inclusion of standard. A case report form (crf), is a printed, optical or electronic document designed to record all of the protocol required information to be reported to the sponsor on each trial subject,. It may be a paper. 1 introduction, background and purpose. The purpose of this sop is to describe the procedure to be. Case Report Form Design.
From www.sampletemplates.com
FREE 12+ Sample Case Report Templates in PDF MS Word Google Docs Case Report Form Design Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. The necessity of incorporating data sharing requirements. 1 introduction, background and purpose. The purpose of this standard operating procedure (sop) is to describe the standard procedures to be followed when. 1.2 the case report form (crf) is a data collection tool used. Case Report Form Design.
From www.greenlight.guru
Electronic Case Report Form (eCRF) How to Design for Medical Device Case Report Form Design Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. A case report form (crf), is a printed, optical or electronic document designed to record all of the protocol required information to be reported to the sponsor on each trial subject,. The purpose of this standard operating procedure (sop) is to describe. Case Report Form Design.
From www.sampletemplates.com
FREE 12+ Sample Case Report Templates in PDF MS Word Google Docs Case Report Form Design It may be a paper. The purpose of this standard operating procedure (sop) is to describe the standard procedures to be followed when. Definitions case report form (crf) form on which individual participant data required by the study protocol are recorded. The purpose of this sop is to describe the procedure to be followed when designing, using and. 1 introduction,. Case Report Form Design.