In A Redox Reaction A Potentiometer Is Used To Measure . potentiometry of redox solutions attempts to measure the open circuit potential of a system. Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. in potentiometry we measure the potential of an electrochemical cell under static conditions. An example of this type of titration would be. 4.04 redox potentials from potentiometric titrations. it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert). potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium.
from www.doeeet.com
it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert). An example of this type of titration would be. Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. 4.04 redox potentials from potentiometric titrations. in potentiometry we measure the potential of an electrochemical cell under static conditions. potentiometry of redox solutions attempts to measure the open circuit potential of a system. potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium.
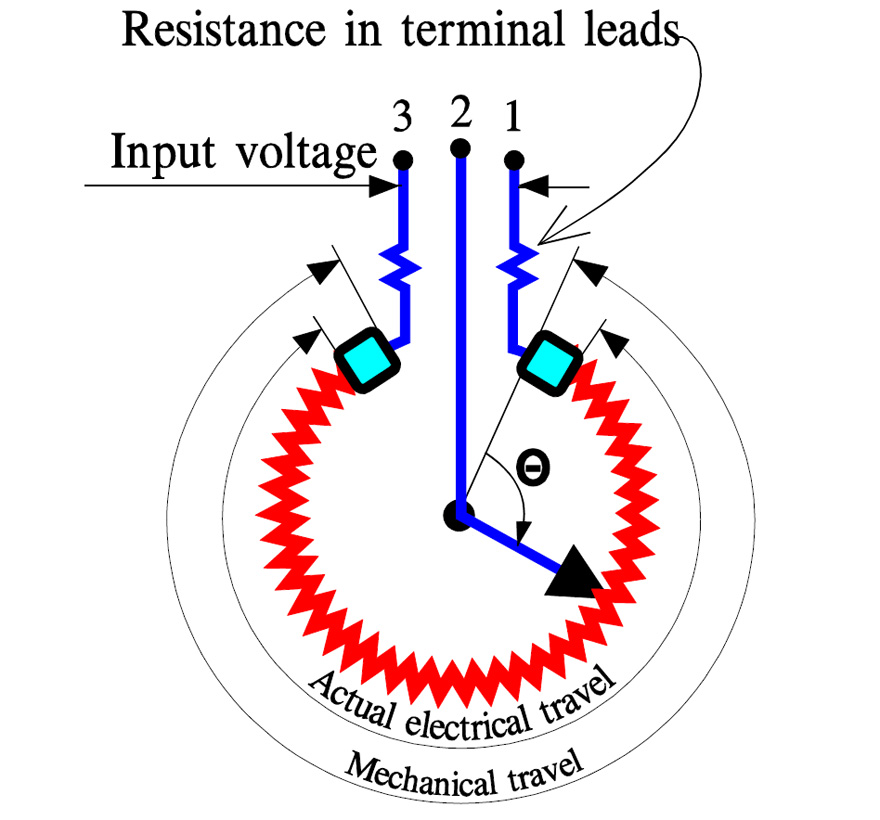
Basic Principles of Potentiometers/Variable Resistors
In A Redox Reaction A Potentiometer Is Used To Measure 4.04 redox potentials from potentiometric titrations. 4.04 redox potentials from potentiometric titrations. This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. in potentiometry we measure the potential of an electrochemical cell under static conditions. potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. potentiometry of redox solutions attempts to measure the open circuit potential of a system. An example of this type of titration would be. it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert).
From ceias.nau.edu
Potentiometry In A Redox Reaction A Potentiometer Is Used To Measure potentiometry of redox solutions attempts to measure the open circuit potential of a system. in potentiometry we measure the potential of an electrochemical cell under static conditions. it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert). An example of this type of titration would be. This type. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.ebay.com
ORP Oxidation Reduction Potentiometer ORP Electrode Measuring Redox Potentia NEW eBay In A Redox Reaction A Potentiometer Is Used To Measure it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert). potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. potentiometry of redox solutions attempts to measure the open circuit potential of a system. Metal electrodes can be. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.ebay.com
ORP Oxidation Reduction Potentiometer ORP Electrode Measuring Redox Potentia NEW eBay In A Redox Reaction A Potentiometer Is Used To Measure 4.04 redox potentials from potentiometric titrations. in potentiometry we measure the potential of an electrochemical cell under static conditions. potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. This type of potentiometric. In A Redox Reaction A Potentiometer Is Used To Measure.
From wiringfixlicensures.z5.web.core.windows.net
Circuit Diagram Of Potentiometer In A Redox Reaction A Potentiometer Is Used To Measure potentiometry of redox solutions attempts to measure the open circuit potential of a system. An example of this type of titration would be. it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert). 4.04 redox potentials from potentiometric titrations. in potentiometry we measure the potential of an. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.researchgate.net
A) Standard redox potentials (E 0 ) for half reactions that can occur... Download Scientific In A Redox Reaction A Potentiometer Is Used To Measure Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. potentiometry of redox solutions attempts to measure the open circuit potential of a system. An example of this type of titration would be. This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. 4.04 redox potentials from potentiometric titrations.. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and reduction.Simple In A Redox Reaction A Potentiometer Is Used To Measure 4.04 redox potentials from potentiometric titrations. it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert). potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. This type of potentiometric titration involves an analyte and titrant that undergo. In A Redox Reaction A Potentiometer Is Used To Measure.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry for Majors In A Redox Reaction A Potentiometer Is Used To Measure This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. potentiometry of redox solutions attempts to measure the open circuit potential of a system. Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. 4.04 redox potentials from potentiometric titrations. An example of this type of titration would be.. In A Redox Reaction A Potentiometer Is Used To Measure.
From dxotohjbd.blob.core.windows.net
What Is Potentiometer Basic at Mathew Plourde blog In A Redox Reaction A Potentiometer Is Used To Measure in potentiometry we measure the potential of an electrochemical cell under static conditions. This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. potentiometry of redox solutions attempts to measure the open circuit potential of a system. 4.04 redox potentials from potentiometric titrations. it responds to a redox reaction at the. In A Redox Reaction A Potentiometer Is Used To Measure.
From opentextbc.ca
Applications of Redox Reactions Voltaic Cells Introductory Chemistry 1st Canadian Edition In A Redox Reaction A Potentiometer Is Used To Measure Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. An example of this type of titration would be. potentiometry of redox solutions attempts to measure the open circuit potential of a system. in potentiometry we measure the potential of an electrochemical cell under static conditions. potentiometric methods are based upon measurements of. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.numerade.com
SOLVED Draw a neat labelled diagram of a simple potentiometer used to measure the emf of a cell In A Redox Reaction A Potentiometer Is Used To Measure in potentiometry we measure the potential of an electrochemical cell under static conditions. This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. 4.04 redox potentials from potentiometric titrations. potentiometry of redox solutions attempts to measure the open circuit potential of a system. potentiometric methods are based upon measurements of the. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.slideserve.com
PPT Redox Reactions in Metabolism Standard reduction potentials, coenzymes in metabolism, and In A Redox Reaction A Potentiometer Is Used To Measure 4.04 redox potentials from potentiometric titrations. An example of this type of titration would be. This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. potentiometry of redox solutions attempts to measure the open circuit potential of a system.. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.slideserve.com
PPT Potentiometry PowerPoint Presentation, free download ID5410570 In A Redox Reaction A Potentiometer Is Used To Measure This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert). 4.04 redox potentials from potentiometric titrations. potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable. In A Redox Reaction A Potentiometer Is Used To Measure.
From studylib.net
4. Potentiometry In A Redox Reaction A Potentiometer Is Used To Measure 4.04 redox potentials from potentiometric titrations. potentiometry of redox solutions attempts to measure the open circuit potential of a system. This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. An example of this type of titration would be. in potentiometry we measure the potential of an electrochemical cell under static conditions.. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.ebay.com
ORP Oxidation Reduction Potentiometer ORP Electrode Measuring The Redox eBay In A Redox Reaction A Potentiometer Is Used To Measure This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. in potentiometry we measure the potential of an electrochemical cell under static conditions. 4.04 redox potentials from potentiometric titrations. Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. An example of this type of titration would be. . In A Redox Reaction A Potentiometer Is Used To Measure.
From www.youtube.com
Potentiometer Instrument Used in Physical Chemistry Part III B. Sc. M. Sc. YouTube In A Redox Reaction A Potentiometer Is Used To Measure it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert). in potentiometry we measure the potential of an electrochemical cell under static conditions. Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. potentiometry of redox solutions attempts to measure the open circuit potential. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.ebay.com
New Orp Oxidation Reduction Potentiometer Orp Electrode Measuring The Redox eBay In A Redox Reaction A Potentiometer Is Used To Measure An example of this type of titration would be. it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert). This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. in potentiometry we measure the potential of an electrochemical cell under static conditions. potentiometry. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.youtube.com
What is a potentiometer. How does a potentiometer work. Potentiometer how it works. YouTube In A Redox Reaction A Potentiometer Is Used To Measure This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. in potentiometry we measure the potential of an electrochemical cell under static conditions. potentiometry of redox solutions attempts to measure the open. In A Redox Reaction A Potentiometer Is Used To Measure.
From chemwiki.ucdavis.edu
11B Potentiometric Methods Chemwiki In A Redox Reaction A Potentiometer Is Used To Measure 4.04 redox potentials from potentiometric titrations. in potentiometry we measure the potential of an electrochemical cell under static conditions. it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert). potentiometry of redox solutions attempts to measure the open circuit potential of a system. This type of potentiometric. In A Redox Reaction A Potentiometer Is Used To Measure.
From chem.libretexts.org
14.2 Electric Potentials in the Cell Chemistry LibreTexts In A Redox Reaction A Potentiometer Is Used To Measure 4.04 redox potentials from potentiometric titrations. potentiometry of redox solutions attempts to measure the open circuit potential of a system. in potentiometry we measure the potential of an electrochemical cell under static conditions. Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. potentiometric methods are based upon measurements of the potential. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.etechnophiles.com
Beginners Guide to Potentiometer Types, Principle, Symbol & Uses In A Redox Reaction A Potentiometer Is Used To Measure potentiometry of redox solutions attempts to measure the open circuit potential of a system. An example of this type of titration would be. it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert). 4.04 redox potentials from potentiometric titrations. potentiometric methods are based upon measurements of the. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.youtube.com
CHEM203 Experiment 6 Redox Potentiometric Titration YouTube In A Redox Reaction A Potentiometer Is Used To Measure potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. An example of this type of titration would be. in potentiometry we measure the potential of an electrochemical cell under static conditions. 4.04 redox potentials from potentiometric titrations. it responds to a redox reaction at the. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.bonanza.com
New ORP Oxidation Reduction Potentiometer ORP Electrode Measuring the Redox Pote Power Tools In A Redox Reaction A Potentiometer Is Used To Measure Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. 4.04 redox potentials from potentiometric titrations. potentiometry of redox solutions attempts to measure the open circuit potential of a system. it responds. In A Redox Reaction A Potentiometer Is Used To Measure.
From alevelchemistry.co.uk
Redox and Electrode Potential ALevel Chemistry Revision Notes In A Redox Reaction A Potentiometer Is Used To Measure it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert). An example of this type of titration would be. 4.04 redox potentials from potentiometric titrations. potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. in potentiometry. In A Redox Reaction A Potentiometer Is Used To Measure.
From makeabilitylab.github.io
L4 Potentiometers Physical Computing In A Redox Reaction A Potentiometer Is Used To Measure in potentiometry we measure the potential of an electrochemical cell under static conditions. it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert). Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. potentiometric methods are based upon measurements of the potential of electrochemical. In A Redox Reaction A Potentiometer Is Used To Measure.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts In A Redox Reaction A Potentiometer Is Used To Measure potentiometry of redox solutions attempts to measure the open circuit potential of a system. This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.aliexpress.com
New ORP Oxidation Reduction Potentiometer ORP Electrode Measuring the Redox Potential Free In A Redox Reaction A Potentiometer Is Used To Measure An example of this type of titration would be. it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert). 4.04 redox potentials from potentiometric titrations. potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. This type of. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.ebay.com
ORP Oxidation Reduction Potentiometer ORP Electrode Measuring Redox Potentia eBay In A Redox Reaction A Potentiometer Is Used To Measure potentiometry of redox solutions attempts to measure the open circuit potential of a system. This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. 4.04 redox potentials from potentiometric titrations. in potentiometry we measure the potential of an electrochemical cell under static conditions. it responds to a redox reaction at the. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.renhotecic.com
PH Meter ORP Oxidation Reduction Potentiometer ORP Electrode Measuring The Redox Potential In A Redox Reaction A Potentiometer Is Used To Measure An example of this type of titration would be. in potentiometry we measure the potential of an electrochemical cell under static conditions. it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert). potentiometry of redox solutions attempts to measure the open circuit potential of a system. This type. In A Redox Reaction A Potentiometer Is Used To Measure.
From studylib.net
Electrodes and Potentiometry Introduction 1.) Potentiometry In A Redox Reaction A Potentiometer Is Used To Measure potentiometry of redox solutions attempts to measure the open circuit potential of a system. Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. it responds to a redox reaction at the metal surface and does not participate in many chemical reactions (inert). An example of this type of titration would be. potentiometric. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.youtube.com
Emf of a cell using potentiometer Current Electricity Class 12 YouTube In A Redox Reaction A Potentiometer Is Used To Measure An example of this type of titration would be. 4.04 redox potentials from potentiometric titrations. Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. This type of potentiometric titration involves an analyte and. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.ebay.com
ORP Oxidation Reduction Potentiometer ORP Electrode Measuring The Redox eBay In A Redox Reaction A Potentiometer Is Used To Measure in potentiometry we measure the potential of an electrochemical cell under static conditions. potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. potentiometry of redox solutions attempts to measure the open circuit potential of a system. Metal electrodes can be used for various redox reactions in. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.numerade.com
SOLVED 8) What is the difference between E and E0 for a redox reaction? Which one runs down to In A Redox Reaction A Potentiometer Is Used To Measure in potentiometry we measure the potential of an electrochemical cell under static conditions. potentiometry of redox solutions attempts to measure the open circuit potential of a system. This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.researchgate.net
Redox potentials of some oxygen reduction and water oxidation reactions. Download Scientific In A Redox Reaction A Potentiometer Is Used To Measure Metal electrodes can be used for various redox reactions in potentiometric titrations and measurements. in potentiometry we measure the potential of an electrochemical cell under static conditions. This type of potentiometric titration involves an analyte and titrant that undergo a redox reaction. potentiometry of redox solutions attempts to measure the open circuit potential of a system. potentiometric. In A Redox Reaction A Potentiometer Is Used To Measure.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts In A Redox Reaction A Potentiometer Is Used To Measure potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. An example of this type of titration would be. in potentiometry we measure the potential of an electrochemical cell under static conditions. it responds to a redox reaction at the metal surface and does not participate in. In A Redox Reaction A Potentiometer Is Used To Measure.
From www.doeeet.com
Basic Principles of Potentiometers/Variable Resistors In A Redox Reaction A Potentiometer Is Used To Measure 4.04 redox potentials from potentiometric titrations. potentiometric methods are based upon measurements of the potential of electrochemical cells in the absence of appreciable currents (an equilibrium. in potentiometry we measure the potential of an electrochemical cell under static conditions. it responds to a redox reaction at the metal surface and does not participate in many chemical. In A Redox Reaction A Potentiometer Is Used To Measure.