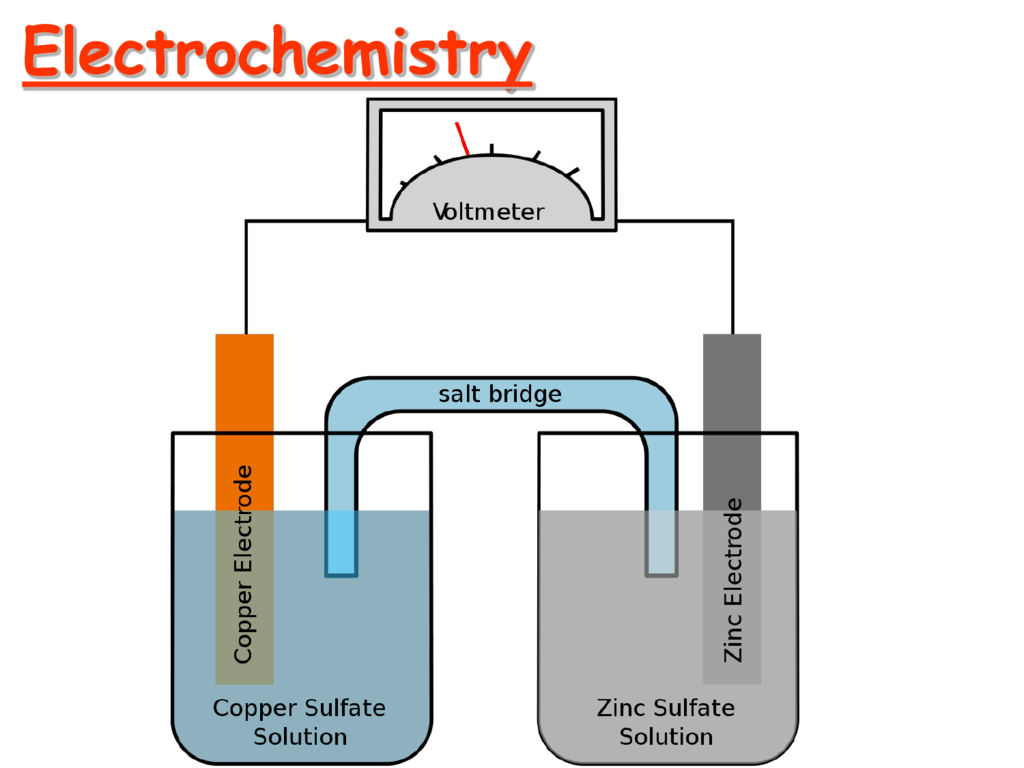
Electrochemistry Applications . Electrochemical processes are used in many ways and their use is likely to increase because they can replace polluting chemical situations. Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological. Describe the basic components of electrochemical cells. Provide a general description of a fuel cell. The principles of cells are used to make electrical batteries. Classify batteries as primary or secondary. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication, nanotechnology,. List some of the characteristics, applications and limitations of. List some of the characteristics and limitations of batteries. Electrochemistry is the study of electricity and how it relates to chemical reactions. Electrochemistry has a number of different uses, particularly in industry.
from studylib.net
The principles of cells are used to make electrical batteries. List some of the characteristics, applications and limitations of. Electrochemistry has a number of different uses, particularly in industry. List some of the characteristics and limitations of batteries. Describe the basic components of electrochemical cells. Provide a general description of a fuel cell. Electrochemical processes are used in many ways and their use is likely to increase because they can replace polluting chemical situations. Classify batteries as primary or secondary. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication, nanotechnology,. Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological.
Introduction to Electrochemistry
Electrochemistry Applications Electrochemical processes are used in many ways and their use is likely to increase because they can replace polluting chemical situations. The principles of cells are used to make electrical batteries. List some of the characteristics and limitations of batteries. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication, nanotechnology,. Electrochemistry has a number of different uses, particularly in industry. Electrochemistry is the study of electricity and how it relates to chemical reactions. Provide a general description of a fuel cell. Classify batteries as primary or secondary. Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological. Describe the basic components of electrochemical cells. Electrochemical processes are used in many ways and their use is likely to increase because they can replace polluting chemical situations. List some of the characteristics, applications and limitations of.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID1195570 Electrochemistry Applications The principles of cells are used to make electrical batteries. List some of the characteristics, applications and limitations of. Electrochemistry is the study of electricity and how it relates to chemical reactions. Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological. Classify batteries as primary or secondary. List. Electrochemistry Applications.
From slideplayer.com
Unit 8 Electrochemistry Applications ppt download Electrochemistry Applications Electrochemistry is the study of electricity and how it relates to chemical reactions. Classify batteries as primary or secondary. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication, nanotechnology,. Electrochemical processes are used in many ways and their use is likely to increase because they can replace polluting chemical situations. Provide a general description. Electrochemistry Applications.
From www.youtube.com
Electrochemistry Applications Batteries YouTube Electrochemistry Applications The principles of cells are used to make electrical batteries. Electrochemistry has a number of different uses, particularly in industry. Provide a general description of a fuel cell. Electrochemical processes are used in many ways and their use is likely to increase because they can replace polluting chemical situations. List some of the characteristics and limitations of batteries. Describe the. Electrochemistry Applications.
From www.slideshare.net
Organic electrochemistry applications Electrochemistry Applications List some of the characteristics and limitations of batteries. The principles of cells are used to make electrical batteries. Classify batteries as primary or secondary. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication, nanotechnology,. Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical. Electrochemistry Applications.
From studylib.net
Introduction to Electrochemistry Electrochemistry Applications List some of the characteristics, applications and limitations of. Electrochemical processes are used in many ways and their use is likely to increase because they can replace polluting chemical situations. Classify batteries as primary or secondary. Electrochemistry is the study of electricity and how it relates to chemical reactions. List some of the characteristics and limitations of batteries. Electrochemistry has. Electrochemistry Applications.
From www.studocu.com
Unit XI Electrochemistry Applications Unit XI Electrochemistry Applications only works with Electrochemistry Applications Electrochemistry has a number of different uses, particularly in industry. Classify batteries as primary or secondary. The principles of cells are used to make electrical batteries. Provide a general description of a fuel cell. Electrochemistry is the study of electricity and how it relates to chemical reactions. List some of the characteristics, applications and limitations of. Electrochemistry is a truly. Electrochemistry Applications.
From www.pw.live
Electrochemistry, Concepts And Applications Electrochemistry Applications Describe the basic components of electrochemical cells. The principles of cells are used to make electrical batteries. Electrochemistry is the study of electricity and how it relates to chemical reactions. List some of the characteristics and limitations of batteries. List some of the characteristics, applications and limitations of. Electrochemistry is a truly multidisciplinary science which can be applied to a. Electrochemistry Applications.
From www.studypool.com
SOLUTION Electrochemistry and applications Studypool Electrochemistry Applications Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological. Provide a general description of a fuel cell. Electrochemistry has a number of different uses, particularly in industry. List some of the characteristics, applications and limitations of. Electrochemistry is the study of electricity and how it relates to chemical. Electrochemistry Applications.
From www.mdpi.com
Nanomaterials Free FullText Advances in Electrochemical Energy Devices Constructed with Electrochemistry Applications The principles of cells are used to make electrical batteries. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication, nanotechnology,. Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological. Electrochemistry is the study of electricity and how it relates to chemical reactions.. Electrochemistry Applications.
From www.slideshare.net
Electrochemistry Applications Physical Electrochemistry Electrochemistry Applications Electrochemistry has a number of different uses, particularly in industry. Electrochemistry is the study of electricity and how it relates to chemical reactions. Provide a general description of a fuel cell. The principles of cells are used to make electrical batteries. Describe the basic components of electrochemical cells. List some of the characteristics and limitations of batteries. Electrochemistry is a. Electrochemistry Applications.
From protonstalk.com
Electrochemical Series Features, Applications, Examples ProtonsTalk Electrochemistry Applications The principles of cells are used to make electrical batteries. List some of the characteristics, applications and limitations of. List some of the characteristics and limitations of batteries. Describe the basic components of electrochemical cells. Electrochemistry has a number of different uses, particularly in industry. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication,. Electrochemistry Applications.
From saylordotorg.github.io
Electrochemistry Electrochemistry Applications Electrochemistry is the study of electricity and how it relates to chemical reactions. Electrochemistry has a number of different uses, particularly in industry. Classify batteries as primary or secondary. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication, nanotechnology,. Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields. Electrochemistry Applications.
From www.slideserve.com
PPT Electrochemistry and Its Applications PowerPoint Presentation, free download ID6210731 Electrochemistry Applications The principles of cells are used to make electrical batteries. List some of the characteristics and limitations of batteries. Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological. Describe the basic components of electrochemical cells. Electrochemical processes are used in many ways and their use is likely to. Electrochemistry Applications.
From saylordotorg.github.io
Electrochemistry Electrochemistry Applications Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication, nanotechnology,. Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological. Electrochemistry is the study of electricity and how it relates to chemical reactions. Provide a general description of a fuel cell. Classify batteries. Electrochemistry Applications.
From mungfali.com
Applications Of Electrochemical Series Electrochemistry Applications Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication, nanotechnology,. Classify batteries as primary or secondary. Describe the basic components of electrochemical cells. Electrochemistry is the study of electricity and how it relates to chemical reactions. List some of the characteristics and limitations of batteries. Electrochemistry is a truly multidisciplinary science which can be. Electrochemistry Applications.
From saylordotorg.github.io
Electrochemistry Electrochemistry Applications Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication, nanotechnology,. Classify batteries as primary or secondary. Provide a general description of a fuel cell. Electrochemistry has a number of different uses, particularly in. Electrochemistry Applications.
From www.youtube.com
Electrochemical Series and its Applications [Year1] YouTube Electrochemistry Applications Describe the basic components of electrochemical cells. Electrochemical processes are used in many ways and their use is likely to increase because they can replace polluting chemical situations. Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological. The principles of cells are used to make electrical batteries. Electrochemistry. Electrochemistry Applications.
From www.eurekalert.org
Seven years of carbonbased electrochemical c EurekAlert! Electrochemistry Applications The principles of cells are used to make electrical batteries. Electrochemistry has a number of different uses, particularly in industry. Describe the basic components of electrochemical cells. Electrochemical processes are used in many ways and their use is likely to increase because they can replace polluting chemical situations. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields. Electrochemistry Applications.
From www.youtube.com
Lesson 10 Applications of Electrochemistry YouTube Electrochemistry Applications Electrochemical processes are used in many ways and their use is likely to increase because they can replace polluting chemical situations. Electrochemistry is the study of electricity and how it relates to chemical reactions. List some of the characteristics, applications and limitations of. List some of the characteristics and limitations of batteries. The principles of cells are used to make. Electrochemistry Applications.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Electrochemistry Applications Describe the basic components of electrochemical cells. Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological. Electrochemistry is the study of electricity and how it relates to chemical reactions. Classify batteries as primary or secondary. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such. Electrochemistry Applications.
From saylordotorg.github.io
Electrochemistry Electrochemistry Applications Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological. Describe the basic components of electrochemical cells. Electrochemistry is the study of electricity and how it relates to chemical reactions. Classify batteries as primary or secondary. Provide a general description of a fuel cell. List some of the characteristics. Electrochemistry Applications.
From worksheetmagicsantos.z13.web.core.windows.net
Electrochemical Cells A Level Chemistry Electrochemistry Applications Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological. Electrochemistry is the study of electricity and how it relates to chemical reactions. Classify batteries as primary or secondary. Electrochemistry has a number of different uses, particularly in industry. Electrochemistry applications refer to the utilization of electrochemical reactions in. Electrochemistry Applications.
From www.cell.com
Optical and Electrochemical Sensors and Biosensors for the Detection of Quinolones Trends in Electrochemistry Applications List some of the characteristics and limitations of batteries. Provide a general description of a fuel cell. Electrochemical processes are used in many ways and their use is likely to increase because they can replace polluting chemical situations. Electrochemistry is the study of electricity and how it relates to chemical reactions. Electrochemistry is a truly multidisciplinary science which can be. Electrochemistry Applications.
From www.researchgate.net
(PDF) MXene chemistry, electrochemistry and energy storage applications Electrochemistry Applications The principles of cells are used to make electrical batteries. Electrochemistry has a number of different uses, particularly in industry. Classify batteries as primary or secondary. Electrochemistry is the study of electricity and how it relates to chemical reactions. List some of the characteristics, applications and limitations of. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields. Electrochemistry Applications.
From www.youtube.com
Electrochemistry Made Easy Commercial Applications of Electrolysis Episode 13 YouTube Electrochemistry Applications Electrochemical processes are used in many ways and their use is likely to increase because they can replace polluting chemical situations. Electrochemistry is the study of electricity and how it relates to chemical reactions. Electrochemistry has a number of different uses, particularly in industry. The principles of cells are used to make electrical batteries. Electrochemistry is a truly multidisciplinary science. Electrochemistry Applications.
From www.youtube.com
Uses of Electrochemistry in our daily life (Electrochemistry part 2 for CBSE class 12 JEE IIT Electrochemistry Applications Electrochemistry is the study of electricity and how it relates to chemical reactions. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication, nanotechnology,. Electrochemistry has a number of different uses, particularly in industry. The principles of cells are used to make electrical batteries. List some of the characteristics, applications and limitations of. List some. Electrochemistry Applications.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID235007 Electrochemistry Applications Electrochemistry is the study of electricity and how it relates to chemical reactions. Describe the basic components of electrochemical cells. List some of the characteristics, applications and limitations of. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication, nanotechnology,. Classify batteries as primary or secondary. List some of the characteristics and limitations of batteries.. Electrochemistry Applications.
From chemistryhall.com
The Best Electrochemistry Book [2023 Review Guide] Electrochemistry Applications Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication, nanotechnology,. Describe the basic components of electrochemical cells. Electrochemical processes are used in many ways and their use is likely to increase because they can replace polluting chemical situations. List some of the characteristics, applications and limitations of. The principles of cells are used to. Electrochemistry Applications.
From chemistry-europe.onlinelibrary.wiley.com
Electrochemical Synthesis Methods of Frameworks and Their Environmental Analysis Electrochemistry Applications Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological. Describe the basic components of electrochemical cells. Electrochemical processes are used in many ways and their use is likely to increase because they can replace polluting chemical situations. List some of the characteristics, applications and limitations of. Classify batteries. Electrochemistry Applications.
From www.researchgate.net
General routes in electrochemical applications. Download Scientific Diagram Electrochemistry Applications Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication, nanotechnology,. Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological. Describe the basic components of electrochemical cells. Electrochemistry has a number of different uses, particularly in industry. The principles of cells are used. Electrochemistry Applications.
From www.slideserve.com
PPT Chapter 19 Electrochemistry PowerPoint Presentation, free download ID651713 Electrochemistry Applications The principles of cells are used to make electrical batteries. Electrochemical processes are used in many ways and their use is likely to increase because they can replace polluting chemical situations. Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological. Classify batteries as primary or secondary. Electrochemistry has. Electrochemistry Applications.
From saylordotorg.github.io
Electrochemistry Electrochemistry Applications Provide a general description of a fuel cell. Electrochemistry has a number of different uses, particularly in industry. List some of the characteristics, applications and limitations of. The principles of cells are used to make electrical batteries. Classify batteries as primary or secondary. Electrochemical processes are used in many ways and their use is likely to increase because they can. Electrochemistry Applications.
From dokumen.tips
(PPT) Electrochemistry Applications of Redox AP Chemistry Chapter 20 Notes DOKUMEN.TIPS Electrochemistry Applications Electrochemistry is a truly multidisciplinary science which can be applied to a variety of fields within the physical, chemical and biological. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication, nanotechnology,. Classify batteries as primary or secondary. List some of the characteristics, applications and limitations of. List some of the characteristics and limitations of. Electrochemistry Applications.
From mungfali.com
Glass Electrode Diagram Electrochemistry Applications Describe the basic components of electrochemical cells. Classify batteries as primary or secondary. Provide a general description of a fuel cell. Electrochemistry has a number of different uses, particularly in industry. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as nanofabrication, nanotechnology,. Electrochemistry is the study of electricity and how it relates to chemical reactions.. Electrochemistry Applications.
From www.researchgate.net
(PDF) Electrochemistry Applications I Electrochemistry Applications Electrochemistry is the study of electricity and how it relates to chemical reactions. Provide a general description of a fuel cell. List some of the characteristics, applications and limitations of. Describe the basic components of electrochemical cells. List some of the characteristics and limitations of batteries. Electrochemistry applications refer to the utilization of electrochemical reactions in various fields such as. Electrochemistry Applications.