Energy Required To Cool Water . The relative humidity of the air is 70% at the start and 100% at the end of the cooling process. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's. One watt is defined as 1 j/s. Calculate the cooling of water in three ways: Specific enthalpy is a measure of the total energy in a unit mass. The formula to calculate water cooling energy (q) is expressed as: From the psychrometric chart we estimate the water enthalpy in the hot air to be 18.7 btu /lb. \ [ q = \dot {m} \times c \times \delta t \] where: Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. Mixing cold and hot water; This means that it takes 4,200 j to raise the. How to cool water to any temperature: The energy required to heat or cool water depends on the temperature difference. Does it take more energy to heat or cool water?
from apollo.nvu.vsc.edu
This means that it takes 4,200 j to raise the. From the psychrometric chart we estimate the water enthalpy in the hot air to be 18.7 btu /lb. Does it take more energy to heat or cool water? Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's. The relative humidity of the air is 70% at the start and 100% at the end of the cooling process. How to cool water to any temperature: It is easy to apply newton's law of cooling with our calculator. \ [ q = \dot {m} \times c \times \delta t \] where: The formula to calculate water cooling energy (q) is expressed as: The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c).
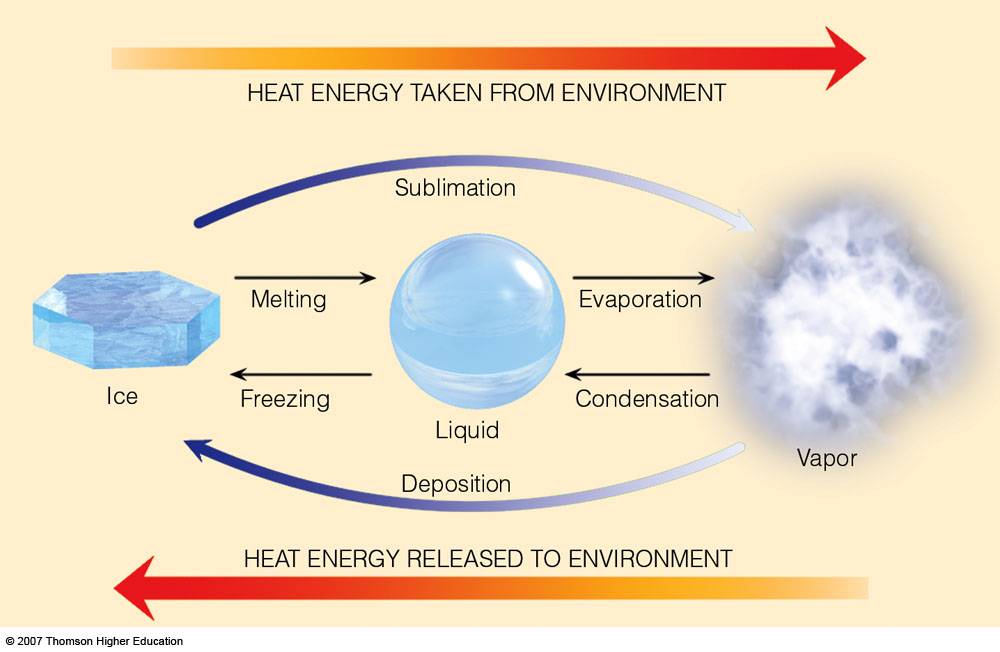
Latent Heats sublimation and deposition
Energy Required To Cool Water The formula to calculate water cooling energy (q) is expressed as: \ [ q = \dot {m} \times c \times \delta t \] where: Calculate the cooling of water in three ways: Mixing cold and hot water; The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). The relative humidity of the air is 70% at the start and 100% at the end of the cooling process. This means that it takes 4,200 j to raise the. It is easy to apply newton's law of cooling with our calculator. How to cool water to any temperature: From the psychrometric chart we estimate the water enthalpy in the hot air to be 18.7 btu /lb. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. One watt is defined as 1 j/s. The energy required to heat or cool water depends on the temperature difference. Specific enthalpy is a measure of the total energy in a unit mass. The formula to calculate water cooling energy (q) is expressed as: Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's.
From www.youtube.com
Energy required reach the boiling temperature of water or to reach 100 Energy Required To Cool Water Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. Calculate the cooling of water in three ways: Does it take more energy to heat or cool water? From the psychrometric chart we estimate the water enthalpy in the hot air. Energy Required To Cool Water.
From www.researchgate.net
Process Flow Schematic for Wet Recirculating Cooling Water System Energy Required To Cool Water The relative humidity of the air is 70% at the start and 100% at the end of the cooling process. Specific enthalpy is a measure of the total energy in a unit mass. How to cool water to any temperature: From the psychrometric chart we estimate the water enthalpy in the hot air to be 18.7 btu /lb. Just specify. Energy Required To Cool Water.
From www.slideserve.com
PPT Heat of Fusion PowerPoint Presentation ID2249917 Energy Required To Cool Water Specific enthalpy is a measure of the total energy in a unit mass. The energy required to heat or cool water depends on the temperature difference. From the psychrometric chart we estimate the water enthalpy in the hot air to be 18.7 btu /lb. Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's. One watt is. Energy Required To Cool Water.
From www.hydro.com.au
How can a dam be a battery? Energy Required To Cool Water Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's. The relative humidity of the air is 70% at the start and 100% at the end of the cooling process. The energy required to heat or cool water depends on the temperature difference. Mixing cold and hot water; Our water heating calculator can help you determine both. Energy Required To Cool Water.
From www.bbc.co.uk
BBC GCSE Bitesize Solar energy Energy Required To Cool Water One watt is defined as 1 j/s. From the psychrometric chart we estimate the water enthalpy in the hot air to be 18.7 btu /lb. Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2. Energy Required To Cool Water.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Energy Required To Cool Water One watt is defined as 1 j/s. Specific enthalpy is a measure of the total energy in a unit mass. This means that it takes 4,200 j to raise the. Calculate the cooling of water in three ways: \ [ q = \dot {m} \times c \times \delta t \] where: The formula to calculate water cooling energy (q) is. Energy Required To Cool Water.
From www.slideshare.net
Energy ch 16 Energy Required To Cool Water \ [ q = \dot {m} \times c \times \delta t \] where: Calculate the cooling of water in three ways: Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's. This means that it takes 4,200 j to raise the. The energy required to heat or cool water depends on the temperature difference. How to cool. Energy Required To Cool Water.
From survival-mastery.com
DIY Hydroelectric Generator You've got the Power! Energy Required To Cool Water The formula to calculate water cooling energy (q) is expressed as: The energy required to heat or cool water depends on the temperature difference. One watt is defined as 1 j/s. Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's. Our water heating calculator can help you determine both the amount of heat required to raise. Energy Required To Cool Water.
From www.sciencelearn.org.nz
Heat and change of state — Science Learning Hub Energy Required To Cool Water The energy required to heat or cool water depends on the temperature difference. The relative humidity of the air is 70% at the start and 100% at the end of the cooling process. How to cool water to any temperature: The formula to calculate water cooling energy (q) is expressed as: \ [ q = \dot {m} \times c \times. Energy Required To Cool Water.
From www.geoexchange.org
Geothermal 101 Geothermal Exchange OrganizationGeothermal Exchange Energy Required To Cool Water The formula to calculate water cooling energy (q) is expressed as: From the psychrometric chart we estimate the water enthalpy in the hot air to be 18.7 btu /lb. It is easy to apply newton's law of cooling with our calculator. The energy required to heat or cool water depends on the temperature difference. The relative humidity of the air. Energy Required To Cool Water.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Energy Required To Cool Water Specific enthalpy is a measure of the total energy in a unit mass. The energy required to heat or cool water depends on the temperature difference. Mixing cold and hot water; How to cool water to any temperature: One watt is defined as 1 j/s. Does it take more energy to heat or cool water? The formula to calculate water. Energy Required To Cool Water.
From www.nuclear-power.com
Cooling System Circulating Water System Energy Required To Cool Water How to cool water to any temperature: This means that it takes 4,200 j to raise the. Specific enthalpy is a measure of the total energy in a unit mass. The relative humidity of the air is 70% at the start and 100% at the end of the cooling process. Calculate the cooling of water in three ways: Our water. Energy Required To Cool Water.
From exyobhadc.blob.core.windows.net
How To Get Air Out Of Water Cooling Loop at Sarah Hughes blog Energy Required To Cool Water Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's. From the psychrometric chart we estimate the water enthalpy in the hot air to be 18.7 btu /lb. The energy required to heat or cool water depends on the temperature difference. Our water heating calculator can help you determine both the amount of heat required to raise. Energy Required To Cool Water.
From www.coursehero.com
[Solved] It is planned to cool water from 110 o F to 85 o F in a packed Energy Required To Cool Water The relative humidity of the air is 70% at the start and 100% at the end of the cooling process. This means that it takes 4,200 j to raise the. Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's. One watt is defined as 1 j/s. Mixing cold and hot water; From the psychrometric chart we. Energy Required To Cool Water.
From www.viridiansolar.co.uk
The different forms of solar energy Energy Required To Cool Water How to cool water to any temperature: One watt is defined as 1 j/s. From the psychrometric chart we estimate the water enthalpy in the hot air to be 18.7 btu /lb. Calculate the cooling of water in three ways: Does it take more energy to heat or cool water? \ [ q = \dot {m} \times c \times \delta. Energy Required To Cool Water.
From www.chegg.com
Solved 14109 A wet cooling tower is to cool 60 kg/s of Energy Required To Cool Water The relative humidity of the air is 70% at the start and 100% at the end of the cooling process. \ [ q = \dot {m} \times c \times \delta t \] where: Mixing cold and hot water; Calculate the cooling of water in three ways: The formula to calculate water cooling energy (q) is expressed as: From the psychrometric. Energy Required To Cool Water.
From haipernews.com
How To Calculate Heat Capacity Of Water Haiper Energy Required To Cool Water Calculate the cooling of water in three ways: The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). It is easy to apply newton's law of cooling with our calculator. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the. Energy Required To Cool Water.
From www.youtube.com
how much ice is needed to cool water? Calculation YouTube Energy Required To Cool Water The relative humidity of the air is 70% at the start and 100% at the end of the cooling process. Mixing cold and hot water; From the psychrometric chart we estimate the water enthalpy in the hot air to be 18.7 btu /lb. The energy required to heat or cool water depends on the temperature difference. Does it take more. Energy Required To Cool Water.
From www.slideserve.com
PPT Heat of Fusion PowerPoint Presentation, free download ID2249917 Energy Required To Cool Water Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's. How to cool water to any temperature: Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. From the psychrometric chart we estimate the water enthalpy in the. Energy Required To Cool Water.
From www.slideserve.com
PPT Phase Changes in Water PowerPoint Presentation, free download Energy Required To Cool Water This means that it takes 4,200 j to raise the. Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's. It is easy to apply newton's law of cooling with our calculator. Calculate the cooling of water in three ways: Does it take more energy to heat or cool water? How to cool water to any temperature:. Energy Required To Cool Water.
From www.chegg.com
Solved 4) Water at 45°C enters a cooling tower (Fig. 3) Energy Required To Cool Water One watt is defined as 1 j/s. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. From the psychrometric chart we estimate the water enthalpy in the hot air to be 18.7 btu /lb. The specific heat capacity of water. Energy Required To Cool Water.
From nca2014.globalchange.gov
Energy, Water, and Land National Climate Assessment Energy Required To Cool Water The relative humidity of the air is 70% at the start and 100% at the end of the cooling process. Specific enthalpy is a measure of the total energy in a unit mass. Mixing cold and hot water; Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's. It is easy to apply newton's law of cooling. Energy Required To Cool Water.
From ecampusontario.pressbooks.pub
Part 4 Cooling Water Systems 2B2 PEG 3725 Power Plant Systems Energy Required To Cool Water Calculate the cooling of water in three ways: Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. From the psychrometric chart we estimate the water enthalpy in the hot air to be 18.7 btu /lb. Mixing cold and hot water;. Energy Required To Cool Water.
From netsolwater.com
How to treat Cooling Tower water Netsol Water Energy Required To Cool Water How to cool water to any temperature: Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's. One watt is defined as 1 j/s. This means that it takes 4,200 j to raise the. Calculate the cooling of water in three ways: \ [ q = \dot {m} \times c \times \delta t \] where: It is. Energy Required To Cool Water.
From www.youtube.com
Water Cooling Explained How It Works and What Parts You Need YouTube Energy Required To Cool Water Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). It is easy. Energy Required To Cool Water.
From news.energysage.com
Solar Water Heating Is It Worth It in 2018? EnergySage Energy Required To Cool Water Mixing cold and hot water; Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. Specific enthalpy is a measure of the total energy in a unit mass. One watt is defined as 1 j/s. The energy required to heat or. Energy Required To Cool Water.
From www.chegg.com
Solved 3) Cooling water leaves the condenser of a power Energy Required To Cool Water It is easy to apply newton's law of cooling with our calculator. Specific enthalpy is a measure of the total energy in a unit mass. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. How to cool water to any. Energy Required To Cool Water.
From exyjuxlrl.blob.core.windows.net
Evaporative Cooling System Architecture at Leonard Delaney blog Energy Required To Cool Water The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). This means that it takes 4,200 j to raise the. It is easy to apply newton's law of cooling with our calculator. \ [ q = \dot {m} \times c \times \delta t \] where: One watt is defined as 1 j/s. The formula to. Energy Required To Cool Water.
From apollo.nvu.vsc.edu
Latent Heats sublimation and deposition Energy Required To Cool Water The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). Specific enthalpy is a measure of the total energy in a unit mass. It is easy to apply newton's law of cooling with our calculator. How to cool water to any temperature: Does it take more energy to heat or cool water? This means that. Energy Required To Cool Water.
From www.slideserve.com
PPT Basic Cooling Water Treatment principles PowerPoint Presentation Energy Required To Cool Water Does it take more energy to heat or cool water? Mixing cold and hot water; One watt is defined as 1 j/s. From the psychrometric chart we estimate the water enthalpy in the hot air to be 18.7 btu /lb. The specific heat capacity of water is 4,200 joules per kilogram per degree celsius (j/kg°c). The formula to calculate water. Energy Required To Cool Water.
From coolingchiwayake.blogspot.com
Cooling Cooling Equation Energy Required To Cool Water This means that it takes 4,200 j to raise the. How to cool water to any temperature: Does it take more energy to heat or cool water? One watt is defined as 1 j/s. Mixing cold and hot water; The energy required to heat or cool water depends on the temperature difference. It is easy to apply newton's law of. Energy Required To Cool Water.
From www.dreamstime.com
The WaterEnergy Nexus Image of a Renewable Energy Project and Its Energy Required To Cool Water Specific enthalpy is a measure of the total energy in a unit mass. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. One watt is defined as 1 j/s. Just specify the initial temperature (let's say 100 °c), the ambient. Energy Required To Cool Water.
From www.britannica.com
Closedcycle OTEC system energy technology Britannica Energy Required To Cool Water Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's. This means that it takes 4,200 j to raise the. The energy required to heat or cool water depends on the temperature difference. Mixing cold and hot water; The relative humidity of the air is 70% at the start and 100% at the end of the cooling. Energy Required To Cool Water.
From sites.psu.edu
About Energy Required To Cool Water How to cool water to any temperature: The formula to calculate water cooling energy (q) is expressed as: This means that it takes 4,200 j to raise the. Just specify the initial temperature (let's say 100 °c), the ambient temperature (let's. The energy required to heat or cool water depends on the temperature difference. Mixing cold and hot water; From. Energy Required To Cool Water.
From www.toppr.com
The heat energy required to raise the temperature of 2kg of water from Energy Required To Cool Water How to cool water to any temperature: Specific enthalpy is a measure of the total energy in a unit mass. From the psychrometric chart we estimate the water enthalpy in the hot air to be 18.7 btu /lb. Our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2. Energy Required To Cool Water.