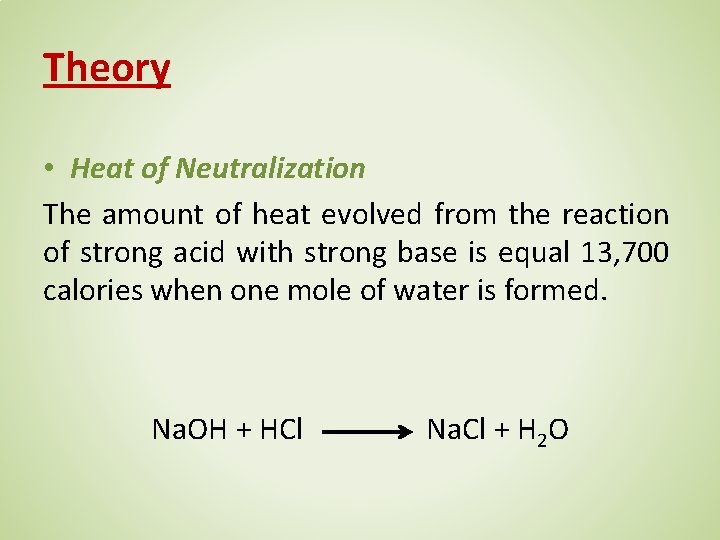Definition Heat Of Neutralization . Recognize if an acid or base is strong or weak. The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; The heat (or enthalpy) of neutralization (∆h) is the heat evolved when an acid and a base react to form a salt plus water. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. 1 hno 2(aq) + naoh. In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. The enthalpy of neutralization is the heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to.
from slidetodoc.com
The heat (or enthalpy) of neutralization (∆h) is the heat evolved when an acid and a base react to form a salt plus water. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. 1 hno 2(aq) + naoh. The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; The enthalpy of neutralization is the heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to. Recognize if an acid or base is strong or weak.
Experiment 2 Determination of the Heat of Neutralization
Definition Heat Of Neutralization The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; 1 hno 2(aq) + naoh. Recognize if an acid or base is strong or weak. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; The enthalpy of neutralization is the heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to. In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. The heat (or enthalpy) of neutralization (∆h) is the heat evolved when an acid and a base react to form a salt plus water.
From www.aplustopper.com
What is the enthalpy of neutralization? A Plus Topper Definition Heat Of Neutralization The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. Recognize if an acid or base is strong or weak. The enthalpy of neutralization is the heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to. In chemistry and. Definition Heat Of Neutralization.
From www.chemistrylearner.com
Heat (Enthalpy) of Neutralization Definition and Formula Definition Heat Of Neutralization The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. 1 hno 2(aq) + naoh. The heat (or enthalpy) of neutralization (∆h) is the heat evolved when an acid and a base react to form a salt plus water. In chemistry and thermodynamics, the enthalpy of neutralization (δh. Definition Heat Of Neutralization.
From chem2u.blogspot.com
chem2U Heat of Neutralisation Definition Heat Of Neutralization In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; Recognize if an acid or base is strong or weak. The standard enthalpy change of neutralisation. Definition Heat Of Neutralization.
From www.slideshare.net
Heat of neutralization Definition Heat Of Neutralization The heat (or enthalpy) of neutralization (∆h) is the heat evolved when an acid and a base react to form a salt plus water. Recognize if an acid or base is strong or weak. The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; In chemistry and thermodynamics, the enthalpy. Definition Heat Of Neutralization.
From www.aplustopper.com
What is the enthalpy of neutralization? A Plus Topper Definition Heat Of Neutralization In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. Recognize if an acid or base is strong or weak. The enthalpy of neutralization is the heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to. The. Definition Heat Of Neutralization.
From www.abcworksheet.com
What is a Neutralization Reaction Definition Definition Heat Of Neutralization Recognize if an acid or base is strong or weak. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. The enthalpy of neutralization is the heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to. In chemistry and. Definition Heat Of Neutralization.
From www.teachoo.com
Neutralization Reaction Definition, Equation and Examples Teachoo Definition Heat Of Neutralization The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. Recognize if an acid or base is strong or weak. The standard enthalpy change of neutralisation. Definition Heat Of Neutralization.
From www.youtube.com
Enthalpy of neutralization YouTube Definition Heat Of Neutralization The heat (or enthalpy) of neutralization (∆h) is the heat evolved when an acid and a base react to form a salt plus water. 1 hno 2(aq) + naoh. In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. The standard enthalpy change of neutralisation is. Definition Heat Of Neutralization.
From study.com
Neutralization Reaction Definition, Equation & Examples Lesson Definition Heat Of Neutralization The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; The enthalpy of neutralization is the heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to. In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy. Definition Heat Of Neutralization.
From dewwool.com
25 Examples of neutralization reaction DewWool Definition Heat Of Neutralization Recognize if an acid or base is strong or weak. 1 hno 2(aq) + naoh. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. The heat (or enthalpy) of neutralization (∆h) is the heat evolved when an acid and a base react to form a salt plus. Definition Heat Of Neutralization.
From www.slideserve.com
PPT Heat of Neutralization PowerPoint Presentation, free download ID5493357 Definition Heat Of Neutralization Recognize if an acid or base is strong or weak. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. The heat (or enthalpy). Definition Heat Of Neutralization.
From slidetodoc.com
Enthalpy Changes Chapter 6 Standards 5 1 5 Definition Heat Of Neutralization The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. The meaning of heat of neutralization is the heat of reaction resulting from the. Definition Heat Of Neutralization.
From www.slideshare.net
Heat of neutralization Definition Heat Of Neutralization The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. The enthalpy of neutralization is the heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to. The meaning of heat of neutralization is the heat of reaction resulting from. Definition Heat Of Neutralization.
From www.chemicals.co.uk
What is Neutralisation in Chemistry? The Chemistry Blog Definition Heat Of Neutralization 1 hno 2(aq) + naoh. The heat (or enthalpy) of neutralization (∆h) is the heat evolved when an acid and a base react to form a salt plus water. In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. Recognize if an acid or base is. Definition Heat Of Neutralization.
From www.studypool.com
SOLUTION How you find heat of neutralization of HCl and NaOH Solution? Studypool Definition Heat Of Neutralization The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. The meaning of heat of neutralization is the heat of reaction resulting from the. Definition Heat Of Neutralization.
From www.slideserve.com
PPT Heat of Neutralization PowerPoint Presentation, free download ID5493357 Definition Heat Of Neutralization Recognize if an acid or base is strong or weak. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. The heat (or enthalpy) of neutralization (∆h) is the heat evolved when an acid and a base react to form a salt plus water. The meaning of heat. Definition Heat Of Neutralization.
From www.slideshare.net
Heat of neutralization Definition Heat Of Neutralization In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. The heat (or enthalpy) of neutralization (∆h) is the heat evolved when an acid and a base react to form a salt plus water. Recognize if an acid or base is strong or weak. The standard. Definition Heat Of Neutralization.
From childhealthpolicy.vumc.org
⭐ Heat of neutralization formula. Neutralization Reaction. 20221007 Definition Heat Of Neutralization Recognize if an acid or base is strong or weak. The heat (or enthalpy) of neutralization (∆h) is the heat evolved when an acid and a base react to form a salt plus water. In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. The enthalpy. Definition Heat Of Neutralization.
From www.youtube.com
Heat of Neutralization Part 1 Thermochemistry YouTube Definition Heat Of Neutralization The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. Recognize if an acid or base is strong or weak. The heat (or enthalpy) of neutralization (∆h) is. Definition Heat Of Neutralization.
From studylib.net
Experiment 28 Heat of Neutralization Definition Heat Of Neutralization Recognize if an acid or base is strong or weak. The enthalpy of neutralization is the heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to. The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; 1 hno 2(aq) + naoh.. Definition Heat Of Neutralization.
From www.chemistrylearner.com
Heat (Enthalpy) of Neutralization Definition and Formula Definition Heat Of Neutralization 1 hno 2(aq) + naoh. In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. Recognize if an acid or base is strong or weak. The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; The. Definition Heat Of Neutralization.
From www.slideshare.net
Heat of neutralization Definition Heat Of Neutralization The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; Recognize if an acid or base is strong or weak. 1 hno 2(aq) + naoh. The heat (or enthalpy) of neutralization (∆h) is the heat evolved when an acid and a base react to form a salt plus water. The. Definition Heat Of Neutralization.
From slidetodoc.com
Experiment 2 Determination of the Heat of Neutralization Definition Heat Of Neutralization The enthalpy of neutralization is the heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to. The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; Recognize if an acid or base is strong or weak. The standard enthalpy change of. Definition Heat Of Neutralization.
From lessonlibrarysnotter.z13.web.core.windows.net
How To Complete Neutralization Reactions Definition Heat Of Neutralization The enthalpy of neutralization is the heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to. 1 hno 2(aq) + naoh. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. Recognize if an acid or base is strong. Definition Heat Of Neutralization.
From chem2u.blogspot.com
chem2U Heat of Neutralisation Definition Heat Of Neutralization The enthalpy of neutralization is the heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to. 1 hno 2(aq) + naoh. In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. Recognize if an acid or base. Definition Heat Of Neutralization.
From www.youtube.com
Calculating Heat of Neutralization YouTube Definition Heat Of Neutralization The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. The enthalpy of neutralization is the heat evolved when one equivalent of an acid. Definition Heat Of Neutralization.
From www.teachoo.com
Neutralization Reaction Definition, Equation and Examples Teachoo Definition Heat Of Neutralization The heat (or enthalpy) of neutralization (∆h) is the heat evolved when an acid and a base react to form a salt plus water. 1 hno 2(aq) + naoh. The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; The standard enthalpy change of neutralisation is the enthalpy change when. Definition Heat Of Neutralization.
From www.slideserve.com
PPT Heat of Neutralization PowerPoint Presentation, free download ID5493357 Definition Heat Of Neutralization The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; The enthalpy of neutralization is the heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid. Definition Heat Of Neutralization.
From sciencenotes.org
Neutralization Reaction Definition and Products Definition Heat Of Neutralization In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. The meaning of heat of neutralization is the heat of reaction resulting from the. Definition Heat Of Neutralization.
From www.slideshare.net
Heat of neutralization Definition Heat Of Neutralization The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. The enthalpy of neutralization is the heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to. In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change. Definition Heat Of Neutralization.
From definitionjull.blogspot.com
Definition Of Neutralization In Chemistry definitionjull Definition Heat Of Neutralization The enthalpy of neutralization is the heat evolved when one equivalent of an acid and one equivalent of a base undergo a neutralization reaction to. The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy. Definition Heat Of Neutralization.
From www.youtube.com
Comparing the Heat of Neutralisation Strong Acid & Strong Alkali Vs Weak Acid & Weak Alkali Definition Heat Of Neutralization Recognize if an acid or base is strong or weak. The heat (or enthalpy) of neutralization (∆h) is the heat evolved when an acid and a base react to form a salt plus water. The standard enthalpy change of neutralisation is the enthalpy change when solutions of an acid and an alkali react together under standard. The enthalpy of neutralization. Definition Heat Of Neutralization.
From www.scribd.com
4.4 Understanding Heat of Neutralization Acid Properties Of Water Definition Heat Of Neutralization Recognize if an acid or base is strong or weak. In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; The standard enthalpy change of neutralisation. Definition Heat Of Neutralization.
From www.teachoo.com
Neutralization Reaction Definition, Equation and Examples Teachoo Definition Heat Of Neutralization Recognize if an acid or base is strong or weak. The meaning of heat of neutralization is the heat of reaction resulting from the neutralization of an acid or base; In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. 1 hno 2(aq) + naoh. The. Definition Heat Of Neutralization.
From www.youtube.com
Enthalpy of Neutralisation Calculations // HSC Chemistry YouTube Definition Heat Of Neutralization Recognize if an acid or base is strong or weak. 1 hno 2(aq) + naoh. In chemistry and thermodynamics, the enthalpy of neutralization (δh n) is the change in enthalpy that occurs when one equivalent of an acid and. The enthalpy of neutralization is the heat evolved when one equivalent of an acid and one equivalent of a base undergo. Definition Heat Of Neutralization.