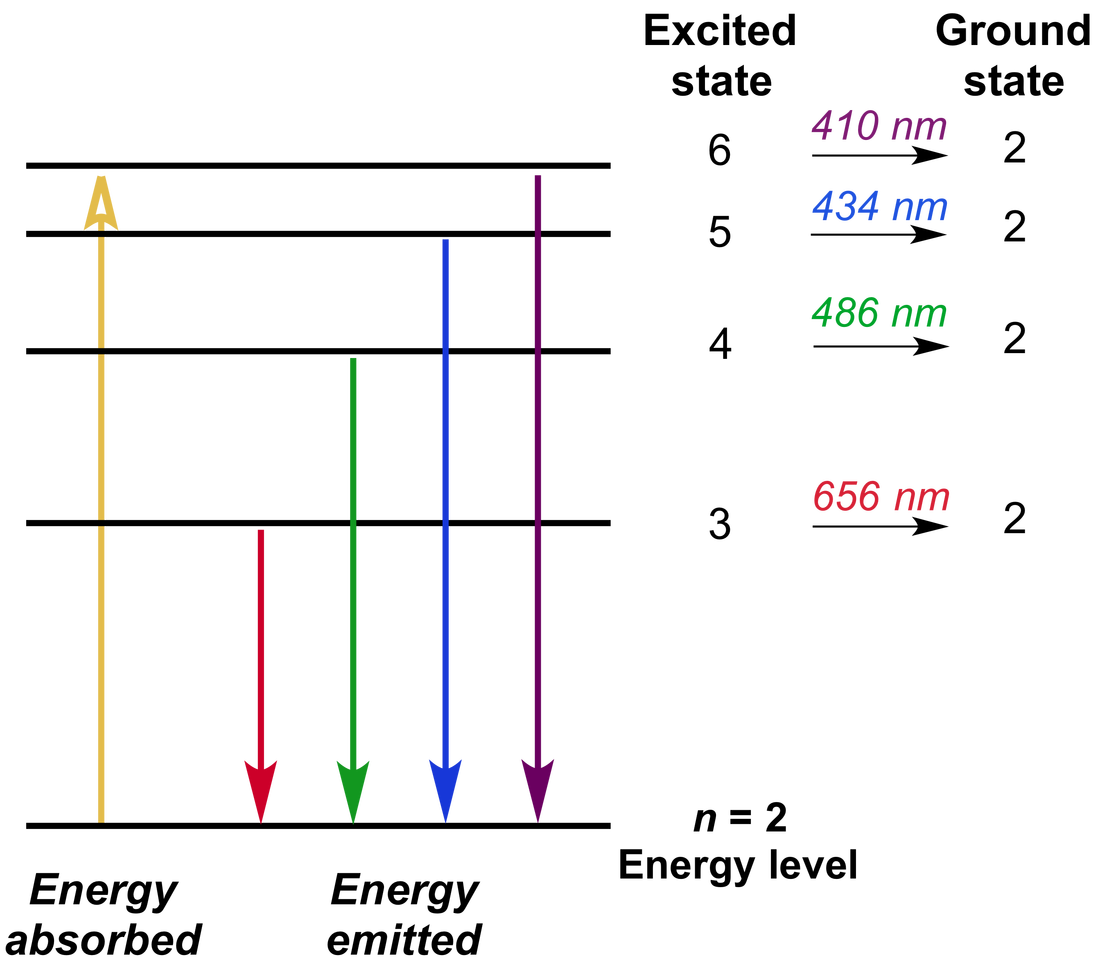
Spectrometry Emission . Atomic emission requires a means for converting a solid, liquid, or solution analyte into a free gaseous atom. The release of a photon following thermal excitation is called emission. Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation state. The focus of this chapter is on the emission of ultraviolet. Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. The same source of thermal energy. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by.
from chemistrypuns-periodically.weebly.com
The same source of thermal energy. The focus of this chapter is on the emission of ultraviolet. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Atomic emission requires a means for converting a solid, liquid, or solution analyte into a free gaseous atom. The release of a photon following thermal excitation is called emission. Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation state.
Chemistry Electron Emission Spectrum
Spectrometry Emission Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. Atomic emission requires a means for converting a solid, liquid, or solution analyte into a free gaseous atom. Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. The focus of this chapter is on the emission of ultraviolet. The same source of thermal energy. Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation state. The release of a photon following thermal excitation is called emission. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum Spectrometry Emission Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. Atomic emission requires a means for converting a solid, liquid, or solution analyte into a free gaseous atom. The same source of thermal energy. The focus of this chapter. Spectrometry Emission.
From medium.com
Light and Dark Understanding Spectrometry by Amelia Settembre Medium Spectrometry Emission Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation state. The release of a photon following thermal excitation is called emission. The focus of this chapter is on the emission of ultraviolet. The same source of thermal energy. Atomic emission requires a means for converting a solid, liquid, or solution analyte into. Spectrometry Emission.
From chemistnotes.com
Inductively Coupled Plasma Atomic Emission Spectroscopy Principle Spectrometry Emission The release of a photon following thermal excitation is called emission. Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation state. The same source of thermal energy. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. The result is an emission spectrum that shows the. Spectrometry Emission.
From joihsprvv.blob.core.windows.net
Emission Spectra Bbc Bitesize at Jose Doty blog Spectrometry Emission Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation state. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. The focus of this chapter is on the emission of ultraviolet. The same source of thermal energy. Atomic emission requires a means for converting a solid,. Spectrometry Emission.
From studylib.net
Chapter 10 ATOMIC EMISSION SPECTROMETRY Spectrometry Emission Atomic emission requires a means for converting a solid, liquid, or solution analyte into a free gaseous atom. The release of a photon following thermal excitation is called emission. Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by.. Spectrometry Emission.
From www.researchgate.net
Schematic representation of atomic emission spectroscopy (AES) with Spectrometry Emission Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation state. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. Classical emission spectroscopy is based on excitation of atoms or. Spectrometry Emission.
From www.priyamstudycentre.com
Atomic Emission Spectroscopy Inductively Coupled Plasma Spectrometry Emission The same source of thermal energy. Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. The focus of this chapter is on the emission of ultraviolet. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. The release of a photon following thermal excitation is called emission. Atomic. Spectrometry Emission.
From www.youtube.com
Inductively coupled plasma optical emission spectroscopy (ICPOES Spectrometry Emission The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. Atomic emission requires a means for converting a solid, liquid, or solution analyte into a free gaseous atom. The same source of thermal energy. Atomic emission spectroscopy (aes). Spectrometry Emission.
From www.slideserve.com
PPT Principle of Emission Spectroscopy I PowerPoint Presentation Spectrometry Emission The same source of thermal energy. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. The focus of this chapter is on the emission of ultraviolet. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Atomic emission requires a means for converting a solid, liquid,. Spectrometry Emission.
From www.researchgate.net
CO 2 optical emission spectroscopy (OES) (a) Schematic of the Spectrometry Emission The focus of this chapter is on the emission of ultraviolet. Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. The same source of thermal energy. The release of a photon following thermal excitation is called emission. Atomic emission requires a means for converting a solid, liquid, or solution analyte into a free gaseous. Spectrometry Emission.
From www.slideshare.net
Atomic Spectroscopy Basic Principles and Instruments Spectrometry Emission The same source of thermal energy. The focus of this chapter is on the emission of ultraviolet. Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. The release of a photon following thermal excitation is called emission.. Spectrometry Emission.
From chem.libretexts.org
13.1 The Spectrum Chemistry LibreTexts Spectrometry Emission The focus of this chapter is on the emission of ultraviolet. The same source of thermal energy. Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation state. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Emission spectroscopy is a technique used for elemental. Spectrometry Emission.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Spectrometry Emission The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. The same source of thermal energy. Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation state. The release of a. Spectrometry Emission.
From webbtelescope.org
Absorption and Emission Spectra of Various Elements b Spectrometry Emission The result is an emission spectrum that shows the intensity of emission as a function of wavelength. The release of a photon following thermal excitation is called emission. Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation state. The focus of this chapter is on the emission of ultraviolet. The same source. Spectrometry Emission.
From www.researchgate.net
The emission spectrum taken by the spectrometer (0.5 m) with (grating Spectrometry Emission The focus of this chapter is on the emission of ultraviolet. Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation state. The release of a photon following thermal excitation is called emission. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Emission spectroscopy is. Spectrometry Emission.
From www.youtube.com
ICPAESInductively coupled plasmaAtomic emission spectroscopy Spectrometry Emission Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. The same source of thermal energy. The focus of this chapter is on the emission of ultraviolet. Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation state. The release of a photon following thermal excitation is called. Spectrometry Emission.
From www.researchgate.net
Schematic arrangement of direct current arc optical emission Spectrometry Emission Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. The focus of this chapter is on the emission of ultraviolet. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. Atomic emission requires a means for converting a solid, liquid, or solution analyte into a free gaseous atom.. Spectrometry Emission.
From www.youtube.com
ICPAES Part B What is Atomic Emission Spectrometry (AES)? YouTube Spectrometry Emission The result is an emission spectrum that shows the intensity of emission as a function of wavelength. The release of a photon following thermal excitation is called emission. The same source of thermal energy. Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. Atomic emission spectroscopy (aes) deals with the excitation of atoms or. Spectrometry Emission.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning Spectrometry Emission The focus of this chapter is on the emission of ultraviolet. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. The same source of thermal energy. Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation state. Atomic emission requires a means for converting a solid,. Spectrometry Emission.
From montessorimuddle.org
Emission Spectra How Atoms Emit and Absorb Light Montessori Muddle Spectrometry Emission Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. The focus of this chapter is on the emission of ultraviolet. Atomic emission requires a means for converting a solid, liquid, or solution analyte into a free gaseous atom. The same source of thermal energy. Atomic emission spectroscopy (aes) deals with the excitation of. Spectrometry Emission.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Spectrometry Emission Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation state. Atomic emission requires a means for converting a solid, liquid, or solution analyte into a free gaseous atom. The focus of this chapter is on the emission of ultraviolet. Classical emission spectroscopy is based on excitation of atoms or molecules into higher. Spectrometry Emission.
From www.youtube.com
Atomic Emission Spectroscopy YouTube Spectrometry Emission The result is an emission spectrum that shows the intensity of emission as a function of wavelength. The release of a photon following thermal excitation is called emission. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. The same source of thermal energy. Atomic emission requires a means for converting a solid, liquid,. Spectrometry Emission.
From brainly.com
What is an emission spectrum? Use the Bohr model to explain why the Spectrometry Emission The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. The release of a photon following thermal excitation is called emission. The same source of thermal energy. Emission spectroscopy is a technique used for elemental analyses, commonly. Spectrometry Emission.
From www.vernier.com
Spectrum of Atomic Hydrogen > Experiment 21 from Advanced Physics with Spectrometry Emission The focus of this chapter is on the emission of ultraviolet. Atomic emission requires a means for converting a solid, liquid, or solution analyte into a free gaseous atom. The release of a photon following thermal excitation is called emission. The same source of thermal energy. The result is an emission spectrum that shows the intensity of emission as a. Spectrometry Emission.
From www.researchgate.net
Emission spectra of different light sources (a) incandescent tungsten Spectrometry Emission Atomic emission requires a means for converting a solid, liquid, or solution analyte into a free gaseous atom. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. Atomic emission spectroscopy (aes) deals with the excitation of. Spectrometry Emission.
From www.researchgate.net
Emission spectrometry of air under different frequencies with the Spectrometry Emission The focus of this chapter is on the emission of ultraviolet. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. The same source of thermal energy. The release of a photon following thermal excitation is called emission. Atomic emission requires a means for converting a solid, liquid, or solution analyte into a free. Spectrometry Emission.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ Spectrometry Emission Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. The focus of this chapter is on the emission of ultraviolet. The release of a photon following thermal excitation is called emission. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. Atomic emission requires a means for converting. Spectrometry Emission.
From chem.libretexts.org
10.7 Atomic Emission Spectroscopy Chemistry LibreTexts Spectrometry Emission The same source of thermal energy. Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation state. Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. The release of a photon following thermal excitation is called emission. The result is an emission spectrum that shows the intensity. Spectrometry Emission.
From blogging2.humanities.manchester.ac.uk
ICPOptical Emission Spectrometry Geography Laboratories Spectrometry Emission Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. The focus of this chapter is on the emission of ultraviolet. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. The same source of thermal energy. The release of a photon following thermal excitation is called emission. Atomic. Spectrometry Emission.
From es.slideshare.net
Emission spectroscopy Spectrometry Emission Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. The release of a photon following thermal excitation is called emission. Atomic emission requires a means for converting a solid, liquid, or solution analyte into a free gaseous atom.. Spectrometry Emission.
From chem.libretexts.org
5.5 Atomic Emission Spectra Chemistry LibreTexts Spectrometry Emission The focus of this chapter is on the emission of ultraviolet. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher. Spectrometry Emission.
From physicsopenlab.org
Arc Atomic Emission Spectroscopy PhysicsOpenLab Spectrometry Emission Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. The release of a photon following thermal excitation is called emission. Atomic emission requires a means for converting a solid, liquid, or solution analyte into a free gaseous atom. The focus of this chapter is on the emission of ultraviolet. The same source of. Spectrometry Emission.
From www.slideserve.com
PPT Atomic Emission Spectrometry PowerPoint Presentation, free Spectrometry Emission Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. The same source of thermal energy. Atomic emission spectroscopy (aes) deals with. Spectrometry Emission.
From www.researchgate.net
(a) Emission spectra of CdSe quantum dots with different sizes Source Spectrometry Emission Atomic emission spectroscopy (aes) deals with the excitation of atoms or elementary ions to their higher excitation state. The same source of thermal energy. Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. The release of a photon following thermal excitation is called emission. The result is an emission spectrum that shows the intensity. Spectrometry Emission.
From www.slideserve.com
PPT Principle of Emission Spectroscopy I PowerPoint Presentation Spectrometry Emission The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Emission spectroscopy is a technique used for elemental analyses, commonly employed to determine plating thicknesses. Classical emission spectroscopy is based on excitation of atoms or molecules into higher electronic states by. The same source of thermal energy. Atomic emission requires a means for. Spectrometry Emission.