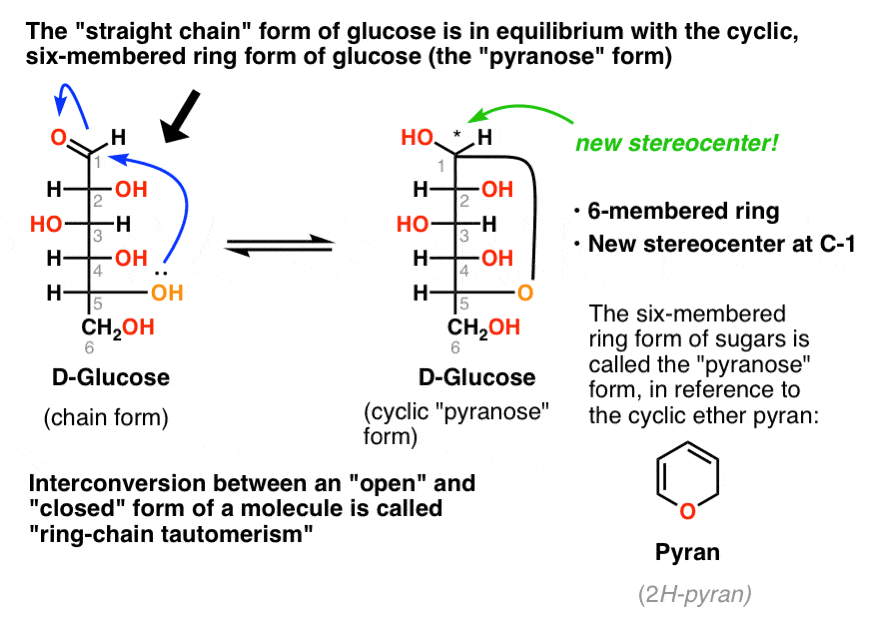
Sugar Ring Chemistry . Sugars in aqueous solution exist in an equilibrium between the linear form and the ring form, which is formed by intramolecular attack by a hydroxyl group on the carbonyl. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. The anomeric carbon atom of a sugar is reactive. Both rings contain an oxygen atom. The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. The anomeric carbon of the ring form of a sugar is the place where most of the chemical action occurs. Well, the anomeric carbon is. You have seen the structure of ribose in the lecture describing the chemistry of atp. Five membered ring sugars are also prevalent in biology. The cyclic form of sugars is the favored form in aqueous solution.
from www.masterorganicchemistry.com
The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. You have seen the structure of ribose in the lecture describing the chemistry of atp. The anomeric carbon of the ring form of a sugar is the place where most of the chemical action occurs. The cyclic form of sugars is the favored form in aqueous solution. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. Both rings contain an oxygen atom. Sugars in aqueous solution exist in an equilibrium between the linear form and the ring form, which is formed by intramolecular attack by a hydroxyl group on the carbonyl. Five membered ring sugars are also prevalent in biology. The anomeric carbon atom of a sugar is reactive. Well, the anomeric carbon is.
Pyranoses and Furanoses RingChain Tautomerism In Sugars — Master
Sugar Ring Chemistry The anomeric carbon of the ring form of a sugar is the place where most of the chemical action occurs. Five membered ring sugars are also prevalent in biology. Sugars in aqueous solution exist in an equilibrium between the linear form and the ring form, which is formed by intramolecular attack by a hydroxyl group on the carbonyl. Well, the anomeric carbon is. The cyclic form of sugars is the favored form in aqueous solution. The anomeric carbon of the ring form of a sugar is the place where most of the chemical action occurs. Both rings contain an oxygen atom. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. You have seen the structure of ribose in the lecture describing the chemistry of atp. The anomeric carbon atom of a sugar is reactive. The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features.
From www.animalia-life.club
Alpha And Beta Glucose Ring Structure Sugar Ring Chemistry The anomeric carbon of the ring form of a sugar is the place where most of the chemical action occurs. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. Five membered ring sugars are also prevalent in biology. The anomeric carbon atom of a sugar is reactive. You. Sugar Ring Chemistry.
From netgroup.edu.vn
Update more than 129 d glucose ring latest netgroup.edu.vn Sugar Ring Chemistry Well, the anomeric carbon is. The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. The anomeric carbon atom of a sugar is reactive. Both. Sugar Ring Chemistry.
From chem.libretexts.org
2.4.1.1 A1. Monosaccharides Structures Chemistry LibreTexts Sugar Ring Chemistry Well, the anomeric carbon is. You have seen the structure of ribose in the lecture describing the chemistry of atp. Five membered ring sugars are also prevalent in biology. The cyclic form of sugars is the favored form in aqueous solution. Sugars in aqueous solution exist in an equilibrium between the linear form and the ring form, which is formed. Sugar Ring Chemistry.
From www.masterorganicchemistry.com
Mutarotation of glucose and other sugars Master Organic Chemistry Sugar Ring Chemistry Five membered ring sugars are also prevalent in biology. The anomeric carbon atom of a sugar is reactive. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. Both rings contain an oxygen atom. The anomeric carbon of the ring form of a sugar is the place where most. Sugar Ring Chemistry.
From www.aliexpress.com
Simple Caffeine/Serotonin/Dopamine/Glucose Sugar Molecule Chemistry Sugar Ring Chemistry The anomeric carbon atom of a sugar is reactive. You have seen the structure of ribose in the lecture describing the chemistry of atp. Both rings contain an oxygen atom. The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. The anomeric carbon of the ring. Sugar Ring Chemistry.
From www.etsy.com
Glucose Sugar Molecule Ring Chemistry by disruptivejewelry Sugar Ring Chemistry You have seen the structure of ribose in the lecture describing the chemistry of atp. The anomeric carbon of the ring form of a sugar is the place where most of the chemical action occurs. Sugars in aqueous solution exist in an equilibrium between the linear form and the ring form, which is formed by intramolecular attack by a hydroxyl. Sugar Ring Chemistry.
From www.sugar.org
What is Sugar? What is Sucrose? The Sugar Association Sugar Ring Chemistry The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. Well, the anomeric carbon is. Five membered ring sugars are also prevalent in biology. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. Sugars. Sugar Ring Chemistry.
From surfguppy.com
3 Simple Steps Draw the ring structure of glucose molecule Sugar Ring Chemistry The anomeric carbon atom of a sugar is reactive. Sugars in aqueous solution exist in an equilibrium between the linear form and the ring form, which is formed by intramolecular attack by a hydroxyl group on the carbonyl. Both rings contain an oxygen atom. The anomeric carbon of the ring form of a sugar is the place where most of. Sugar Ring Chemistry.
From ar.inspiredpencil.com
Glucose Ring Structure Formation Sugar Ring Chemistry Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. The anomeric carbon atom of a sugar is reactive. The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. Sugars in aqueous solution exist in. Sugar Ring Chemistry.
From www.researchgate.net
Types of sugar rings and their interactions with MV. Optimized atomic Sugar Ring Chemistry The anomeric carbon atom of a sugar is reactive. You have seen the structure of ribose in the lecture describing the chemistry of atp. Five membered ring sugars are also prevalent in biology. The cyclic form of sugars is the favored form in aqueous solution. The size of the cyclic hemiacetal ring adopted by a given sugar is not constant,. Sugar Ring Chemistry.
From www.masterorganicchemistry.com
Pyranoses and Furanoses RingChain Tautomerism In Sugars — Master Sugar Ring Chemistry The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. Sugars in aqueous solution exist in an equilibrium between the linear form and the ring form, which is formed by intramolecular attack by a hydroxyl group on the carbonyl. The anomeric carbon atom of a sugar. Sugar Ring Chemistry.
From www.dreamstime.com
Glucose Chemical Formulas. Molecular Structure. Science Element Sugar Ring Chemistry Well, the anomeric carbon is. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. The anomeric carbon atom of a sugar is reactive. You have seen the structure of ribose in the lecture describing the chemistry of atp. Five membered ring sugars are also prevalent in biology. The. Sugar Ring Chemistry.
From www.slideserve.com
PPT Basic organic chemistry of important macromolecules PowerPoint Sugar Ring Chemistry The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. Well, the anomeric carbon is. The anomeric carbon atom of a sugar is reactive. Sugars in aqueous solution exist in an equilibrium between the linear form and the ring form, which is formed by intramolecular attack. Sugar Ring Chemistry.
From www.dbriers.com
Draw the Structure of a Glucose Molecule Sugar Ring Chemistry You have seen the structure of ribose in the lecture describing the chemistry of atp. Well, the anomeric carbon is. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. The anomeric carbon atom of a sugar is reactive. Sugars in aqueous solution exist in an equilibrium between the. Sugar Ring Chemistry.
From www.reagent.co.uk
What are the Different Types of Sugar? ReAgent Chemicals Sugar Ring Chemistry The cyclic form of sugars is the favored form in aqueous solution. Five membered ring sugars are also prevalent in biology. You have seen the structure of ribose in the lecture describing the chemistry of atp. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. The size of. Sugar Ring Chemistry.
From healthjade.com
What is Carbohydrates? Foods, Healthy Carbs for Weight Loss Sugar Ring Chemistry Both rings contain an oxygen atom. The anomeric carbon of the ring form of a sugar is the place where most of the chemical action occurs. The cyclic form of sugars is the favored form in aqueous solution. Sugars in aqueous solution exist in an equilibrium between the linear form and the ring form, which is formed by intramolecular attack. Sugar Ring Chemistry.
From visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry Sugar Ring Chemistry Five membered ring sugars are also prevalent in biology. The anomeric carbon of the ring form of a sugar is the place where most of the chemical action occurs. Both rings contain an oxygen atom. The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. Aldolhexoses. Sugar Ring Chemistry.
From www.youtube.com
B.3.2 Draw the straightchain and ring structural formulas of glucose Sugar Ring Chemistry The cyclic form of sugars is the favored form in aqueous solution. The anomeric carbon of the ring form of a sugar is the place where most of the chemical action occurs. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. Well, the anomeric carbon is. Five membered. Sugar Ring Chemistry.
From www.animalia-life.club
Alpha And Beta Glucose Ring Structure Sugar Ring Chemistry Both rings contain an oxygen atom. Five membered ring sugars are also prevalent in biology. The cyclic form of sugars is the favored form in aqueous solution. The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. The anomeric carbon of the ring form of a. Sugar Ring Chemistry.
From learn.careers360.com
What is Biomolecules Definition of Biomolecules, Notes, Examples, Books Sugar Ring Chemistry The anomeric carbon atom of a sugar is reactive. The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. Well, the anomeric carbon is. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. You. Sugar Ring Chemistry.
From www.columbia.edu
lec3a_00 Sugar Ring Chemistry Well, the anomeric carbon is. The cyclic form of sugars is the favored form in aqueous solution. You have seen the structure of ribose in the lecture describing the chemistry of atp. The anomeric carbon of the ring form of a sugar is the place where most of the chemical action occurs. Sugars in aqueous solution exist in an equilibrium. Sugar Ring Chemistry.
From ar.inspiredpencil.com
Glucose Ring Structure Model Sugar Ring Chemistry The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. The anomeric carbon of the ring form of a sugar is the place where most of the chemical action occurs. Five membered ring sugars are also prevalent in biology. The anomeric carbon atom of a sugar. Sugar Ring Chemistry.
From mavink.com
Beta Glucose Ring Structure Sugar Ring Chemistry Sugars in aqueous solution exist in an equilibrium between the linear form and the ring form, which is formed by intramolecular attack by a hydroxyl group on the carbonyl. The cyclic form of sugars is the favored form in aqueous solution. The anomeric carbon atom of a sugar is reactive. Five membered ring sugars are also prevalent in biology. The. Sugar Ring Chemistry.
From bakerpedia.com
Glucose Baking Ingredients BAKERpedia Sugar Ring Chemistry Sugars in aqueous solution exist in an equilibrium between the linear form and the ring form, which is formed by intramolecular attack by a hydroxyl group on the carbonyl. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. The anomeric carbon of the ring form of a sugar. Sugar Ring Chemistry.
From alevelbiology.co.uk
Glucose Structure, Properties, Synthesis, Facts & Summary Sugar Ring Chemistry You have seen the structure of ribose in the lecture describing the chemistry of atp. The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. Five membered ring sugars are also prevalent in biology. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer. Sugar Ring Chemistry.
From ar.inspiredpencil.com
Glucose Ring Structure Formation Sugar Ring Chemistry Sugars in aqueous solution exist in an equilibrium between the linear form and the ring form, which is formed by intramolecular attack by a hydroxyl group on the carbonyl. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. Both rings contain an oxygen atom. Five membered ring sugars. Sugar Ring Chemistry.
From www.animalia-life.club
Alpha And Beta Glucose Ring Structure Sugar Ring Chemistry The anomeric carbon atom of a sugar is reactive. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. Well, the anomeric carbon is. The cyclic form of sugars is the favored form in aqueous solution. Sugars in aqueous solution exist in an equilibrium between the linear form and. Sugar Ring Chemistry.
From www.suppreviewers.com
The 411 on Dexanhydrous Glucose in Workout Supplements Sugar Ring Chemistry The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. Sugars in aqueous solution exist in an equilibrium between the linear form and the ring form, which is formed by intramolecular attack by a hydroxyl group on the carbonyl. The cyclic form of sugars is the. Sugar Ring Chemistry.
From www.reagent.co.uk
What are the Different Types of Sugar? ReAgent Chemicals Sugar Ring Chemistry You have seen the structure of ribose in the lecture describing the chemistry of atp. The anomeric carbon atom of a sugar is reactive. Well, the anomeric carbon is. The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. The anomeric carbon of the ring form. Sugar Ring Chemistry.
From www.indigoinstruments.com
Molymod Glucose (3 molecules) molecular model kit Sugar Ring Chemistry You have seen the structure of ribose in the lecture describing the chemistry of atp. Well, the anomeric carbon is. The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. Both rings contain an oxygen atom. Sugars in aqueous solution exist in an equilibrium between the. Sugar Ring Chemistry.
From www.chemistrysteps.com
Oxidation of Monosaccharide Carbohydrates Chemistry Steps Sugar Ring Chemistry The anomeric carbon atom of a sugar is reactive. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. Sugars in aqueous solution exist in an equilibrium between the linear form and the ring form, which is formed by intramolecular attack by a hydroxyl group on the carbonyl. Well,. Sugar Ring Chemistry.
From vova.edu.vn
Details more than 62 d glucose ring vova.edu.vn Sugar Ring Chemistry You have seen the structure of ribose in the lecture describing the chemistry of atp. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. The cyclic form of sugars is the favored form in aqueous solution. The anomeric carbon atom of a sugar is reactive. The size of. Sugar Ring Chemistry.
From www.masterorganicchemistry.com
Mutarotation of glucose and other sugars Master Organic Chemistry Sugar Ring Chemistry Five membered ring sugars are also prevalent in biology. The anomeric carbon atom of a sugar is reactive. Both rings contain an oxygen atom. You have seen the structure of ribose in the lecture describing the chemistry of atp. The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and. Sugar Ring Chemistry.
From www.masterorganicchemistry.com
Sugar and Carbohydrate Chemistry Definitions 29 Key Terms To Know Sugar Ring Chemistry The anomeric carbon of the ring form of a sugar is the place where most of the chemical action occurs. Aldolhexoses usually form pyranose rings and their pentose homologs tend to prefer the furanose form, but there are many counter examples. Both rings contain an oxygen atom. The size of the cyclic hemiacetal ring adopted by a given sugar is. Sugar Ring Chemistry.
From forums.studentdoctor.net
L or D Sugar from Ring? Student Doctor Network Sugar Ring Chemistry The anomeric carbon of the ring form of a sugar is the place where most of the chemical action occurs. The size of the cyclic hemiacetal ring adopted by a given sugar is not constant, but may vary with substituents and other structural features. The cyclic form of sugars is the favored form in aqueous solution. You have seen the. Sugar Ring Chemistry.