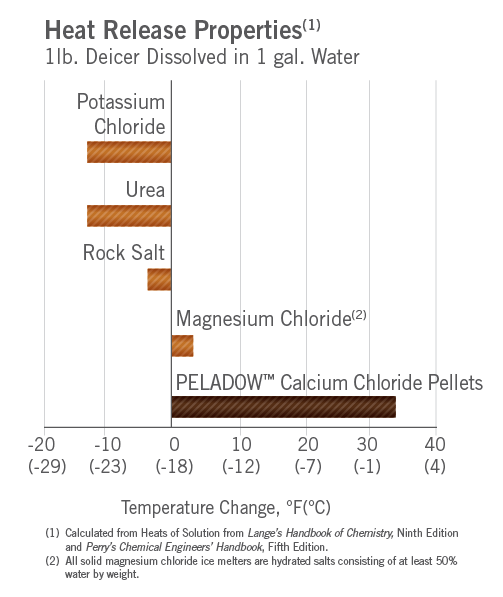
Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride . More ions mean more ions getting in the. In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to form a strong brine, emit heat to. Calcium chloride is more effective at melting ice because it can break down into three ions instead of two: Learn how salt, calcium chloride, magnesium chloride and potassium acetate differ in. Although dissolving calcium chloride liberates. This melting point makes calcium. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). One calcium ion and two chloride ions. Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. When comparing rock salt vs calcium chloride for ice melting, it's important to consider effectiveness and temperature considerations.
from www.oxycalciumchloride.com
More ions mean more ions getting in the. When comparing rock salt vs calcium chloride for ice melting, it's important to consider effectiveness and temperature considerations. One calcium ion and two chloride ions. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). This melting point makes calcium. Calcium chloride is more effective at melting ice because it can break down into three ions instead of two: Learn how salt, calcium chloride, magnesium chloride and potassium acetate differ in. Although dissolving calcium chloride liberates. Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to form a strong brine, emit heat to.
The Case for Calcium Chloride Sidewalk Snow and Ice OxyChem Calcium
Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to form a strong brine, emit heat to. Although dissolving calcium chloride liberates. Calcium chloride is more effective at melting ice because it can break down into three ions instead of two: In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to form a strong brine, emit heat to. When comparing rock salt vs calcium chloride for ice melting, it's important to consider effectiveness and temperature considerations. More ions mean more ions getting in the. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. One calcium ion and two chloride ions. Learn how salt, calcium chloride, magnesium chloride and potassium acetate differ in. This melting point makes calcium.
From www.slideserve.com
PPT What Substance Makes Ice Melt The Fastest? PowerPoint Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride One calcium ion and two chloride ions. When comparing rock salt vs calcium chloride for ice melting, it's important to consider effectiveness and temperature considerations. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to form a. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From snowicesalt.com
What is Calcium Chloride Ice Melt? Snow, Salt, Ice (SISCU) Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Learn how salt, calcium chloride, magnesium chloride and potassium acetate differ in. Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. This melting point makes calcium. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). Calcium chloride is more effective at melting. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From www.youtube.com
ComboTherm Calcium Chloride Ice Melt in ACTION YouTube Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride When comparing rock salt vs calcium chloride for ice melting, it's important to consider effectiveness and temperature considerations. This melting point makes calcium. Calcium chloride is more effective at melting ice because it can break down into three ions instead of two: Although dissolving calcium chloride liberates. In practice, once calcium chloride and magnesium chloride touch ice or snow, they. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From www.nepal.ubuy.com
Buy Snow Joe MELT20ESB Calcium Chloride Ice Melt Blend , 20Pound Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride One calcium ion and two chloride ions. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). Calcium chloride is more effective at melting ice because it can break down into three ions instead of two: In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to form. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From asseenonthe.net
Best Calcium Chloride Ice Melt 6 Choices To Consider As Seen On The Net Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Learn how salt, calcium chloride, magnesium chloride and potassium acetate differ in. This melting point makes calcium. Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. One calcium ion and two chloride ions. Calcium chloride is more effective at melting ice because it can break down into three. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From www.homedepot.com
FDC 15 lbs. Calcium Chloride Ice Melt96CCHLOR15 The Home Depot Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride More ions mean more ions getting in the. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). Calcium chloride is more effective at melting ice because it can break down into three ions instead of two: In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From cleansetec.com
Rock Salt vs Calcium Chloride What is the Difference? Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to form a strong brine, emit heat to. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From ar.inspiredpencil.com
Calcium Chloride Ice Melt Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. More ions mean more ions getting in the. One calcium ion and two chloride ions. When comparing rock salt vs calcium chloride for ice melting, it's important to consider effectiveness and temperature considerations. Learn how salt, calcium chloride, magnesium. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From sciencing.com
How Does Calcium Chloride Melt Ice? Sciencing Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride When comparing rock salt vs calcium chloride for ice melting, it's important to consider effectiveness and temperature considerations. Learn how salt, calcium chloride, magnesium chloride and potassium acetate differ in. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). Although dissolving calcium chloride liberates. This melting point makes calcium. In practice, once calcium chloride. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From www.youtube.com
Calcium Chloride Ice Melt When, Where & How to Use YouTube Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Calcium chloride is more effective at melting ice because it can break down into three ions instead of two: More ions mean more ions getting in the. Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. This melting point makes calcium. One calcium ion and two chloride ions.. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From www.kissner.com
Calcium Chloride Ice Melt Ice Melter Distributor Salt Supplier Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). One calcium ion and two chloride ions. Although dissolving calcium chloride liberates. When comparing rock salt vs calcium chloride for ice melting, it's important to consider effectiveness and temperature considerations. Learn how salt, calcium chloride, magnesium chloride and potassium acetate differ in. Calcium chloride is. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From www.betna-star-salt-chemicals.com
Ice Melting Calcium Chloride. betna star salt & chemicals Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Calcium chloride is more effective at melting ice because it can break down into three ions instead of two: Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to form a strong brine, emit heat to. When. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From ninjadeicer.com
Calcium Chloride vs Calcium Magnesium Acetate Ninja DeIcer Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Although dissolving calcium chloride liberates. More ions mean more ions getting in the. One calcium ion and two chloride ions. Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. This melting point makes calcium. In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From www.lowes.com
Calcium Chloride Ice Melt at Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Although dissolving calcium chloride liberates. One calcium ion and two chloride ions. When comparing rock salt vs calcium chloride for ice melting, it's important to consider effectiveness and temperature considerations. This melting point makes calcium. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). Calcium chloride produces an exothermic reaction when mixed with water. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From earthdevelopmentinc.com
Calcium Chloride vs Sodium Chloride Earth Development Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride One calcium ion and two chloride ions. In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to form a strong brine, emit heat to. Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. Learn how salt, calcium chloride, magnesium chloride. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From www.lowes.com
Bare Ground 7lb Fast Acting Calcium Chloride Ice Melt at Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Although dissolving calcium chloride liberates. In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to form a strong brine, emit heat to. More ions mean more ions getting in the. When comparing rock salt vs calcium chloride for ice melting, it's important to consider effectiveness and temperature considerations. Calcium chloride produces an. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From thekatynews.com
Why Dust And IceMelt Control Services Use Liquid Calcium Chloride For Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride When comparing rock salt vs calcium chloride for ice melting, it's important to consider effectiveness and temperature considerations. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). Learn how salt, calcium chloride, magnesium chloride and potassium acetate differ in. This melting point makes calcium. One calcium ion and two chloride ions. Calcium chloride is. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From periodictable.com
Calcium Chloride ice melter, a sample of the element Calcium in the Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride One calcium ion and two chloride ions. Although dissolving calcium chloride liberates. More ions mean more ions getting in the. When comparing rock salt vs calcium chloride for ice melting, it's important to consider effectiveness and temperature considerations. In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to form a strong brine,. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From snowicesalt.com
Ice Melt Comparison Chart Snow & Ice Salt & Chemicals Unlimited, LLC Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to form a strong brine, emit heat to. When comparing rock salt vs calcium chloride for ice melting, it's important to consider. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From www.oxycalciumchloride.com
The Case for Calcium Chloride Sidewalk Snow and Ice OxyChem Calcium Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. When comparing rock salt vs calcium chloride for ice melting, it's important to consider effectiveness and temperature considerations. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). More ions mean more ions getting. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From flashugnews.com
Comparing Calcium Chloride Ice Melt to Other Deicing Agents Which is Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride More ions mean more ions getting in the. Learn how salt, calcium chloride, magnesium chloride and potassium acetate differ in. Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. Although dissolving calcium chloride liberates. One calcium ion and two chloride ions. When comparing rock salt vs calcium chloride. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From safepaw.com
The Role Of Calcium Chloride In Ice Melting Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride This melting point makes calcium. Although dissolving calcium chloride liberates. One calcium ion and two chloride ions. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). Calcium chloride is more effective at melting ice because it can break down into three ions instead of two: In practice, once calcium chloride and magnesium chloride touch. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From safepaw.com
Why Calcium Chloride is the Ice Melt King Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Learn how salt, calcium chloride, magnesium chloride and potassium acetate differ in. Calcium chloride is more effective at melting ice because it can break down into three ions instead of two: Although dissolving calcium chloride liberates. In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to form a strong brine, emit heat. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From www.factorydirectchemicals.com
96 Pure Calcium Chloride Pellets SNOW & ICE Melter Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. This melting point makes calcium. Although dissolving calcium chloride liberates. Learn how salt, calcium chloride, magnesium chloride and potassium acetate differ in. Calcium chloride is more effective at melting ice because it can break down into three ions instead. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From chemcoindustries.com
Calcium Chloride Ice Melt Pellets ( 50 lb bags) Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride When comparing rock salt vs calcium chloride for ice melting, it's important to consider effectiveness and temperature considerations. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). One calcium ion and two chloride ions. Calcium chloride is more effective at melting ice because it can break down into three ions instead of two: More. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From www.pngegg.com
Calcium chloride Melting Sodium chloride, ice, material, sodium png Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Although dissolving calcium chloride liberates. Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). One calcium ion and two chloride ions. Learn how salt, calcium chloride, magnesium chloride and potassium acetate differ in.. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From ninjadeicer.com
What Is Calcium Chloride Ice melt? Ninja DeIcer Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride More ions mean more ions getting in the. Calcium chloride is more effective at melting ice because it can break down into three ions instead of two: Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). Although dissolving calcium chloride liberates. This melting point makes calcium. When comparing rock salt vs calcium chloride for. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From www.homedepot.com
50 lb. Calcium Chloride Ice Melting Pellets7892 The Home Depot Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). This melting point makes calcium. Calcium chloride is more effective at melting ice because it can break down into three ions instead of two: More ions mean more ions getting in the. Learn how salt, calcium chloride, magnesium chloride and potassium acetate differ in. One. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From favpng.com
Calcium Chloride Chemical Substance Sodium Chloride Melting Chemistry Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Calcium chloride is more effective at melting ice because it can break down into three ions instead of two: Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). Learn how salt, calcium chloride, magnesium chloride and potassium acetate differ in. This melting point makes calcium. In practice, once calcium chloride and magnesium chloride touch. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From www.walmart.com
Scotwood Excel Calcium Chloride Pellets Ice Melt, Melts to 25 Degrees Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to form a strong brine, emit heat to. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From www.chegg.com
Chemistry Archive May 23, 2018 Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to form a strong brine, emit heat to. This melting point makes calcium. More ions mean more ions getting in the. Although dissolving calcium chloride liberates. One calcium ion and two chloride ions. Calcium chloride is more effective at melting ice because it. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From ninjadeicer.com
Rock Salt vs Ice Melt What’s the Difference? Ninja DeIcer Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride One calcium ion and two chloride ions. In practice, once calcium chloride and magnesium chloride touch ice or snow, they immediately pick up water to form a strong brine, emit heat to. This melting point makes calcium. Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. Calcium chloride. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From ldpwatersheds.org
How Does Salt Melt Snow and Ice? LDP Watersheds Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride This melting point makes calcium. Although dissolving calcium chloride liberates. One calcium ion and two chloride ions. More ions mean more ions getting in the. Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From www.walmart.com
Snow Joe MELT Calcium Chloride Crystals Ice Melter (50 lb. Resealable Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride Calcium chloride produces an exothermic reaction when mixed with water or when placed on top of ice, which means that it. Although dissolving calcium chloride liberates. One calcium ion and two chloride ions. Calcium chloride is more effective than sodium chloride in a ratio of 2.8:1 (figure 3a). Calcium chloride is more effective at melting ice because it can break. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.
From castillaestructuras.com
Comparing Calcium Chloride Vs Sodium Chloride For Melting, 52 OFF Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride One calcium ion and two chloride ions. More ions mean more ions getting in the. When comparing rock salt vs calcium chloride for ice melting, it's important to consider effectiveness and temperature considerations. Learn how salt, calcium chloride, magnesium chloride and potassium acetate differ in. This melting point makes calcium. Calcium chloride produces an exothermic reaction when mixed with water. Why Does Calcium Chloride Melt Ice Better Than Sodium Chloride.