Spectroscopy Graph . a spectrum can be displayed as a picture or on a graph. finally, here are your unknown concentrations. learn how spectra show the intensity and energy of light emitted by different objects and elements. The result is a technique known as infrared spectroscopy, which is a useful and quick tool for identifying the bonds present in a given molecule. What is actually being observed spectroscopically is the absorbance of. Different sources of light have different spectra because they have different. They are calculated for you using the system of equations discussed. learn how to use spectra to study the properties and composition of different materials using light. we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed.
from www.jasco-global.com
a spectrum can be displayed as a picture or on a graph. learn how spectra show the intensity and energy of light emitted by different objects and elements. finally, here are your unknown concentrations. What is actually being observed spectroscopically is the absorbance of. we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed. The result is a technique known as infrared spectroscopy, which is a useful and quick tool for identifying the bonds present in a given molecule. They are calculated for you using the system of equations discussed. Different sources of light have different spectra because they have different. learn how to use spectra to study the properties and composition of different materials using light.
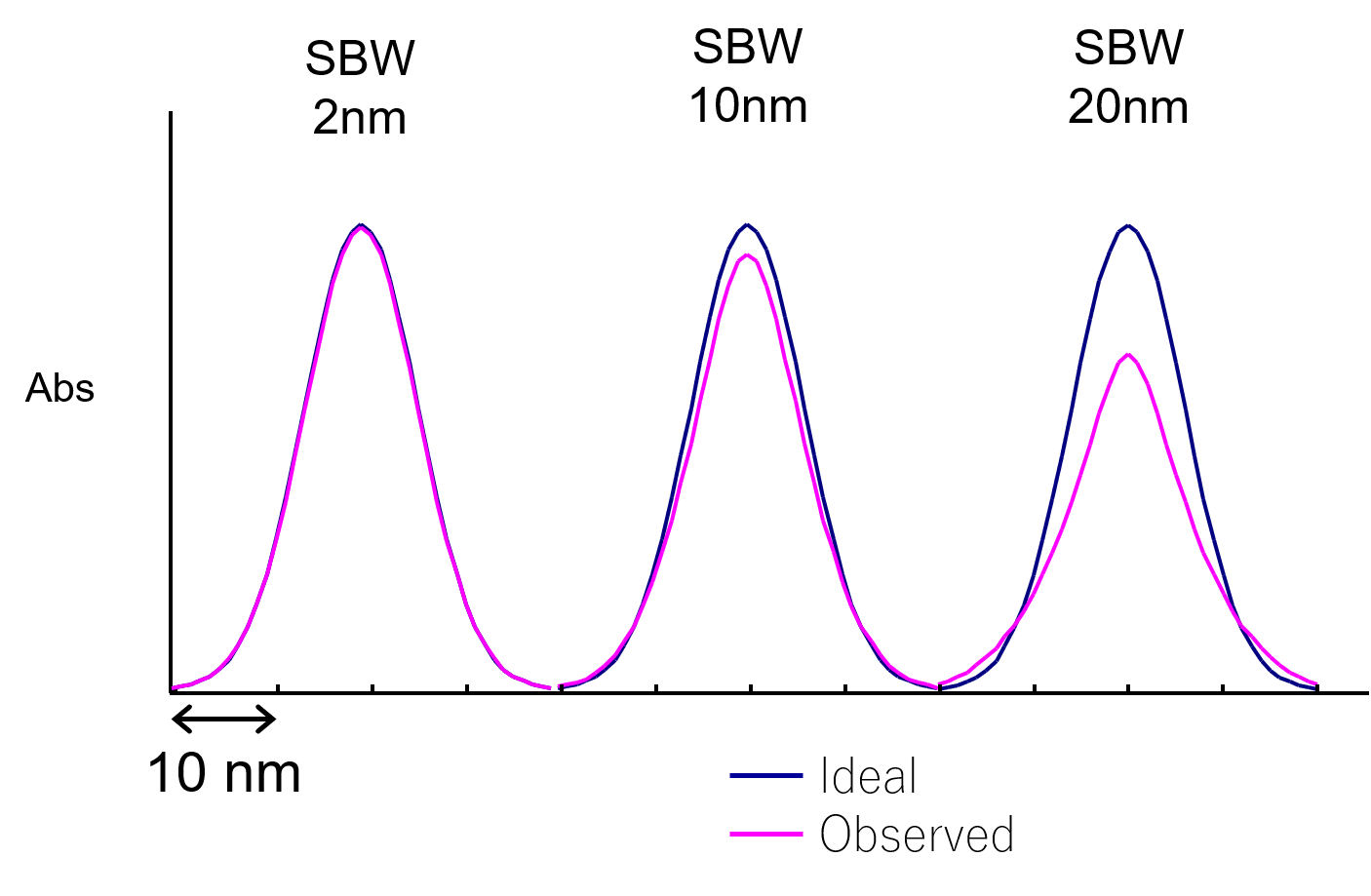
Principles of UV/vis spectroscopy (7) Bandwidth JASCO Global
Spectroscopy Graph finally, here are your unknown concentrations. a spectrum can be displayed as a picture or on a graph. learn how spectra show the intensity and energy of light emitted by different objects and elements. learn how to use spectra to study the properties and composition of different materials using light. What is actually being observed spectroscopically is the absorbance of. Different sources of light have different spectra because they have different. finally, here are your unknown concentrations. The result is a technique known as infrared spectroscopy, which is a useful and quick tool for identifying the bonds present in a given molecule. we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed. They are calculated for you using the system of equations discussed.
From spiff.rit.edu
Spectrographs and Spectra Spectroscopy Graph finally, here are your unknown concentrations. The result is a technique known as infrared spectroscopy, which is a useful and quick tool for identifying the bonds present in a given molecule. we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed. . Spectroscopy Graph.
From dsp.stackexchange.com
Deriving the frequency spectrum graph from the graph of a spectrogram Spectroscopy Graph The result is a technique known as infrared spectroscopy, which is a useful and quick tool for identifying the bonds present in a given molecule. learn how spectra show the intensity and energy of light emitted by different objects and elements. a spectrum can be displayed as a picture or on a graph. Different sources of light have. Spectroscopy Graph.
From www.vernier.com
Use the SpectroVis Plus to Explore Fluorescence Spectroscopy Spectroscopy Graph They are calculated for you using the system of equations discussed. Different sources of light have different spectra because they have different. finally, here are your unknown concentrations. What is actually being observed spectroscopically is the absorbance of. learn how to use spectra to study the properties and composition of different materials using light. a spectrum can. Spectroscopy Graph.
From courses.lumenlearning.com
The Spectrum Astronomy Spectroscopy Graph They are calculated for you using the system of equations discussed. finally, here are your unknown concentrations. The result is a technique known as infrared spectroscopy, which is a useful and quick tool for identifying the bonds present in a given molecule. we can observe and measure this “singing” of bonds by applying ir radiation to a sample. Spectroscopy Graph.
From www.comsol.com
Calculating the Emission Spectra from Common Light Sources COMSOL Blog Spectroscopy Graph Different sources of light have different spectra because they have different. learn how spectra show the intensity and energy of light emitted by different objects and elements. a spectrum can be displayed as a picture or on a graph. we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring. Spectroscopy Graph.
From ibsen.com
Fluorescence Spectrum with PEBBLE VIS Ibsen Photonics Spectroscopy Graph learn how spectra show the intensity and energy of light emitted by different objects and elements. They are calculated for you using the system of equations discussed. What is actually being observed spectroscopically is the absorbance of. The result is a technique known as infrared spectroscopy, which is a useful and quick tool for identifying the bonds present in. Spectroscopy Graph.
From serc.carleton.edu
Infrared and Raman spectroscopy Spectroscopy Graph a spectrum can be displayed as a picture or on a graph. finally, here are your unknown concentrations. learn how spectra show the intensity and energy of light emitted by different objects and elements. learn how to use spectra to study the properties and composition of different materials using light. we can observe and measure. Spectroscopy Graph.
From en.wikipedia.org
Infrared spectroscopy Wikipedia Spectroscopy Graph we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed. They are calculated for you using the system of equations discussed. learn how spectra show the intensity and energy of light emitted by different objects and elements. finally, here are your. Spectroscopy Graph.
From www.youtube.com
Introduction to IR Spectroscopy How to Read an Infrared Spectroscopy Spectroscopy Graph we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed. finally, here are your unknown concentrations. a spectrum can be displayed as a picture or on a graph. They are calculated for you using the system of equations discussed. Different sources. Spectroscopy Graph.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Spectroscopy Graph The result is a technique known as infrared spectroscopy, which is a useful and quick tool for identifying the bonds present in a given molecule. What is actually being observed spectroscopically is the absorbance of. learn how to use spectra to study the properties and composition of different materials using light. learn how spectra show the intensity and. Spectroscopy Graph.
From www.researchgate.net
The spectrum of (a) ultravioletvisible (UVVis) spectrum and (b) curve Spectroscopy Graph learn how spectra show the intensity and energy of light emitted by different objects and elements. What is actually being observed spectroscopically is the absorbance of. learn how to use spectra to study the properties and composition of different materials using light. Different sources of light have different spectra because they have different. a spectrum can be. Spectroscopy Graph.
From www.youtube.com
How to Read A Simple Infrared Spectroscopy (IR Spec) Graph YouTube Spectroscopy Graph Different sources of light have different spectra because they have different. a spectrum can be displayed as a picture or on a graph. They are calculated for you using the system of equations discussed. finally, here are your unknown concentrations. learn how spectra show the intensity and energy of light emitted by different objects and elements. . Spectroscopy Graph.
From clarkgray.hashnode.dev
Raman spectroscopy & AI (Part 2) Spectroscopy Graph learn how to use spectra to study the properties and composition of different materials using light. finally, here are your unknown concentrations. learn how spectra show the intensity and energy of light emitted by different objects and elements. we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring. Spectroscopy Graph.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Ultraviolet spectroscopy Spectroscopy Graph we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed. learn how spectra show the intensity and energy of light emitted by different objects and elements. The result is a technique known as infrared spectroscopy, which is a useful and quick tool. Spectroscopy Graph.
From www.vrogue.co
A Fluorescence Emission Spectra And B Absorption Spec vrogue.co Spectroscopy Graph Different sources of light have different spectra because they have different. They are calculated for you using the system of equations discussed. finally, here are your unknown concentrations. a spectrum can be displayed as a picture or on a graph. learn how spectra show the intensity and energy of light emitted by different objects and elements. The. Spectroscopy Graph.
From www.researchgate.net
Xray photoelectron spectroscopy of 10 nm β and α phase W. Download Spectroscopy Graph a spectrum can be displayed as a picture or on a graph. finally, here are your unknown concentrations. learn how spectra show the intensity and energy of light emitted by different objects and elements. What is actually being observed spectroscopically is the absorbance of. Different sources of light have different spectra because they have different. They are. Spectroscopy Graph.
From tobyrsmith.github.io
Reflectance Spectra Tutorial Spectroscopy Graph The result is a technique known as infrared spectroscopy, which is a useful and quick tool for identifying the bonds present in a given molecule. What is actually being observed spectroscopically is the absorbance of. learn how spectra show the intensity and energy of light emitted by different objects and elements. a spectrum can be displayed as a. Spectroscopy Graph.
From www.jasco-global.com
Principles of UV/vis spectroscopy (7) Bandwidth JASCO Global Spectroscopy Graph What is actually being observed spectroscopically is the absorbance of. finally, here are your unknown concentrations. Different sources of light have different spectra because they have different. learn how to use spectra to study the properties and composition of different materials using light. The result is a technique known as infrared spectroscopy, which is a useful and quick. Spectroscopy Graph.
From chem.libretexts.org
10.1 Overview of Spectroscopy Chemistry LibreTexts Spectroscopy Graph What is actually being observed spectroscopically is the absorbance of. Different sources of light have different spectra because they have different. we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed. a spectrum can be displayed as a picture or on a. Spectroscopy Graph.
From pressbooks.online.ucf.edu
5.3 Spectroscopy in Astronomy Astronomy Spectroscopy Graph a spectrum can be displayed as a picture or on a graph. finally, here are your unknown concentrations. Different sources of light have different spectra because they have different. The result is a technique known as infrared spectroscopy, which is a useful and quick tool for identifying the bonds present in a given molecule. They are calculated for. Spectroscopy Graph.
From www.masterorganicchemistry.com
Interpreting IR Specta A Quick Guide Master Organic Chemistry Spectroscopy Graph we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed. learn how to use spectra to study the properties and composition of different materials using light. finally, here are your unknown concentrations. learn how spectra show the intensity and energy. Spectroscopy Graph.
From rkdmb.home.xs4all.nl
Flame spectroscopy Spectroscopy Graph a spectrum can be displayed as a picture or on a graph. we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed. They are calculated for you using the system of equations discussed. What is actually being observed spectroscopically is the absorbance. Spectroscopy Graph.
From www.researchgate.net
Example of fitting Raman spectra top graph shows measured Raman spect Spectroscopy Graph we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed. Different sources of light have different spectra because they have different. learn how to use spectra to study the properties and composition of different materials using light. What is actually being observed. Spectroscopy Graph.
From chem.libretexts.org
10.6 Photoluminescence Spectroscopy Chemistry LibreTexts Spectroscopy Graph learn how to use spectra to study the properties and composition of different materials using light. finally, here are your unknown concentrations. What is actually being observed spectroscopically is the absorbance of. learn how spectra show the intensity and energy of light emitted by different objects and elements. Different sources of light have different spectra because they. Spectroscopy Graph.
From flexfamous.weebly.com
Atomic spectra of hydrogen atom Flexfamous Spectroscopy Graph Different sources of light have different spectra because they have different. They are calculated for you using the system of equations discussed. finally, here are your unknown concentrations. we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed. learn how spectra. Spectroscopy Graph.
From www.chemistrystudent.com
IR (Infrared Spectroscopy) (ALevel) ChemistryStudent Spectroscopy Graph The result is a technique known as infrared spectroscopy, which is a useful and quick tool for identifying the bonds present in a given molecule. What is actually being observed spectroscopically is the absorbance of. They are calculated for you using the system of equations discussed. Different sources of light have different spectra because they have different. a spectrum. Spectroscopy Graph.
From www.researchgate.net
Raman Spectroscopy graphs of Graphene and GO Spectroscopy Graph we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed. Different sources of light have different spectra because they have different. learn how spectra show the intensity and energy of light emitted by different objects and elements. The result is a technique. Spectroscopy Graph.
From www.vernier.com
A Quantitative Investigation of the Helium Spectrum Spectroscopy Graph What is actually being observed spectroscopically is the absorbance of. learn how to use spectra to study the properties and composition of different materials using light. we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed. learn how spectra show the. Spectroscopy Graph.
From www.chemistrystudent.com
Mass Spectrometry (ALevel) ChemistryStudent Spectroscopy Graph learn how spectra show the intensity and energy of light emitted by different objects and elements. learn how to use spectra to study the properties and composition of different materials using light. The result is a technique known as infrared spectroscopy, which is a useful and quick tool for identifying the bonds present in a given molecule. What. Spectroscopy Graph.
From chem.libretexts.org
4.5 Ultraviolet and visible spectroscopy Chemistry LibreTexts Spectroscopy Graph a spectrum can be displayed as a picture or on a graph. They are calculated for you using the system of equations discussed. What is actually being observed spectroscopically is the absorbance of. finally, here are your unknown concentrations. learn how spectra show the intensity and energy of light emitted by different objects and elements. we. Spectroscopy Graph.
From mavink.com
Ftir Spectroscopy Graph Spectroscopy Graph we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed. finally, here are your unknown concentrations. What is actually being observed spectroscopically is the absorbance of. learn how to use spectra to study the properties and composition of different materials using. Spectroscopy Graph.
From enginemanualmanuel101.z19.web.core.windows.net
Ir Spectrum Guide Spectroscopy Graph a spectrum can be displayed as a picture or on a graph. What is actually being observed spectroscopically is the absorbance of. learn how to use spectra to study the properties and composition of different materials using light. They are calculated for you using the system of equations discussed. Different sources of light have different spectra because they. Spectroscopy Graph.
From chem.libretexts.org
4.5 Ultraviolet and visible spectroscopy Chemistry LibreTexts Spectroscopy Graph learn how spectra show the intensity and energy of light emitted by different objects and elements. The result is a technique known as infrared spectroscopy, which is a useful and quick tool for identifying the bonds present in a given molecule. What is actually being observed spectroscopically is the absorbance of. Different sources of light have different spectra because. Spectroscopy Graph.
From www.researchgate.net
Xray photoelectron spectroscopy (XPS) analysis, (a) full scan spectrum Spectroscopy Graph What is actually being observed spectroscopically is the absorbance of. learn how to use spectra to study the properties and composition of different materials using light. we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed. Different sources of light have different. Spectroscopy Graph.
From mungfali.com
Raman Spectroscopy Graph Spectroscopy Graph learn how spectra show the intensity and energy of light emitted by different objects and elements. we can observe and measure this “singing” of bonds by applying ir radiation to a sample and measuring the frequencies at which the radiation is absorbed. What is actually being observed spectroscopically is the absorbance of. learn how to use spectra. Spectroscopy Graph.