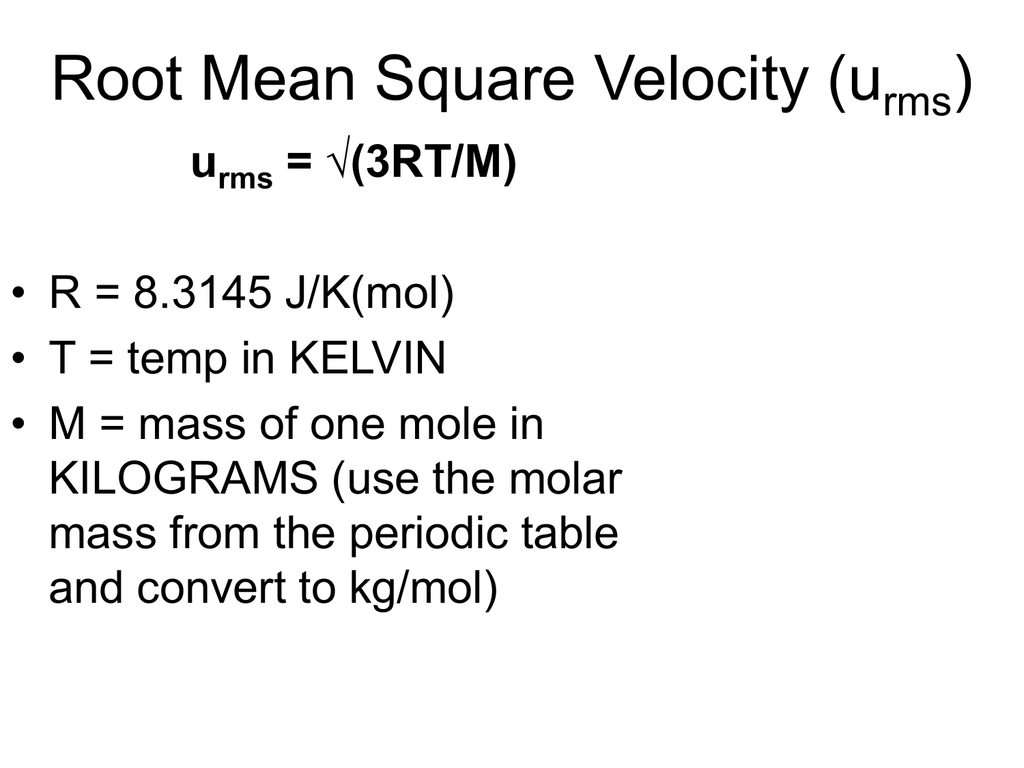
What Is Velocity Constant In Chemistry . In other words, this is when the rate of change of position of an. the rms velocity is directly proportional to the square root of temperature and inversely proportional to the square root of molar mass. It suggests that the velocity (or rate) at which gas molecules move is inversely proportional to the square root of their. the higher the curve at a given speed, the more molecules travel at that speed. For example, many molecules have speeds around 500. this is the equation you need to use: Thus quadrupling the temperature of a given gas doubles the rms velocity of the molecules. V = (3rt) / m. explain the relationships between instantaneous velocity, average velocity, instantaneous speed,. You may, if you wish, read more about the above equation here. if the volume is held constant, the increased speed of the gas molecules results in more frequent and more forceful collisions with. this equation is a modified form of graham's law. a first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one. a velocity is constant when both its magnitude and direction do not change over time. The basic idea is that, if you consider each gas.
from www.tessshebaylo.com
You may, if you wish, read more about the above equation here. a velocity is constant when both its magnitude and direction do not change over time. the higher the curve at a given speed, the more molecules travel at that speed. Thus quadrupling the temperature of a given gas doubles the rms velocity of the molecules. It suggests that the velocity (or rate) at which gas molecules move is inversely proportional to the square root of their. For example, many molecules have speeds around 500. this equation is a modified form of graham's law. In other words, this is when the rate of change of position of an. if the volume is held constant, the increased speed of the gas molecules results in more frequent and more forceful collisions with. explain the relationships between instantaneous velocity, average velocity, instantaneous speed,.
Equation For Velocity Chemistry Tessshebaylo
What Is Velocity Constant In Chemistry The basic idea is that, if you consider each gas. a velocity is constant when both its magnitude and direction do not change over time. this is the equation you need to use: the rms velocity is directly proportional to the square root of temperature and inversely proportional to the square root of molar mass. the higher the curve at a given speed, the more molecules travel at that speed. You may, if you wish, read more about the above equation here. this equation is a modified form of graham's law. It suggests that the velocity (or rate) at which gas molecules move is inversely proportional to the square root of their. if the volume is held constant, the increased speed of the gas molecules results in more frequent and more forceful collisions with. explain the relationships between instantaneous velocity, average velocity, instantaneous speed,. Thus quadrupling the temperature of a given gas doubles the rms velocity of the molecules. V = (3rt) / m. a first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one. In other words, this is when the rate of change of position of an. The basic idea is that, if you consider each gas. For example, many molecules have speeds around 500.
From study.com
Constant Velocity Definition, Equation & Examples Video & Lesson Transcript What Is Velocity Constant In Chemistry the rms velocity is directly proportional to the square root of temperature and inversely proportional to the square root of molar mass. the higher the curve at a given speed, the more molecules travel at that speed. It suggests that the velocity (or rate) at which gas molecules move is inversely proportional to the square root of their.. What Is Velocity Constant In Chemistry.
From www.theengineeringprojects.com
What is Velocity? Definition, SI Unit, Examples & Applications The Engineering Projects What Is Velocity Constant In Chemistry In other words, this is when the rate of change of position of an. a velocity is constant when both its magnitude and direction do not change over time. For example, many molecules have speeds around 500. if the volume is held constant, the increased speed of the gas molecules results in more frequent and more forceful collisions. What Is Velocity Constant In Chemistry.
From www.youtube.com
Molecular Speed of Gases Formula With Boltzmann's Constant YouTube What Is Velocity Constant In Chemistry if the volume is held constant, the increased speed of the gas molecules results in more frequent and more forceful collisions with. the higher the curve at a given speed, the more molecules travel at that speed. this is the equation you need to use: It suggests that the velocity (or rate) at which gas molecules move. What Is Velocity Constant In Chemistry.
From www.slideserve.com
PPT Motion with Constant Velocity in 1D PowerPoint Presentation, free download ID2615973 What Is Velocity Constant In Chemistry The basic idea is that, if you consider each gas. a velocity is constant when both its magnitude and direction do not change over time. You may, if you wish, read more about the above equation here. Thus quadrupling the temperature of a given gas doubles the rms velocity of the molecules. the higher the curve at a. What Is Velocity Constant In Chemistry.
From www.slideserve.com
PPT Motion with Constant Velocity in 1D PowerPoint Presentation, free download ID2615973 What Is Velocity Constant In Chemistry It suggests that the velocity (or rate) at which gas molecules move is inversely proportional to the square root of their. a velocity is constant when both its magnitude and direction do not change over time. In other words, this is when the rate of change of position of an. if the volume is held constant, the increased. What Is Velocity Constant In Chemistry.
From www.youtube.com
Constant Velocity YouTube What Is Velocity Constant In Chemistry explain the relationships between instantaneous velocity, average velocity, instantaneous speed,. this equation is a modified form of graham's law. The basic idea is that, if you consider each gas. You may, if you wish, read more about the above equation here. the higher the curve at a given speed, the more molecules travel at that speed. . What Is Velocity Constant In Chemistry.
From meaningkosh.com
What Does Constant Velocity Mean MeaningKosh What Is Velocity Constant In Chemistry explain the relationships between instantaneous velocity, average velocity, instantaneous speed,. V = (3rt) / m. if the volume is held constant, the increased speed of the gas molecules results in more frequent and more forceful collisions with. the rms velocity is directly proportional to the square root of temperature and inversely proportional to the square root of. What Is Velocity Constant In Chemistry.
From www.fhybea.com
Constant Velocity Motion Examples and Formulas Fhybea What Is Velocity Constant In Chemistry the higher the curve at a given speed, the more molecules travel at that speed. this equation is a modified form of graham's law. The basic idea is that, if you consider each gas. a velocity is constant when both its magnitude and direction do not change over time. In other words, this is when the rate. What Is Velocity Constant In Chemistry.
From www.sliderbase.com
Atomic Structure and Periodicity Presentation Chemistry What Is Velocity Constant In Chemistry In other words, this is when the rate of change of position of an. if the volume is held constant, the increased speed of the gas molecules results in more frequent and more forceful collisions with. the higher the curve at a given speed, the more molecules travel at that speed. For example, many molecules have speeds around. What Is Velocity Constant In Chemistry.
From www.youtube.com
Specific Rate Constant or Velocity Constant, Chemistry Lecture Sabaq.pk YouTube What Is Velocity Constant In Chemistry the rms velocity is directly proportional to the square root of temperature and inversely proportional to the square root of molar mass. if the volume is held constant, the increased speed of the gas molecules results in more frequent and more forceful collisions with. a velocity is constant when both its magnitude and direction do not change. What Is Velocity Constant In Chemistry.
From www.slideserve.com
PPT Derivation of Kinematic Equations PowerPoint Presentation, free download ID172075 What Is Velocity Constant In Chemistry V = (3rt) / m. In other words, this is when the rate of change of position of an. For example, many molecules have speeds around 500. this is the equation you need to use: if the volume is held constant, the increased speed of the gas molecules results in more frequent and more forceful collisions with. It. What Is Velocity Constant In Chemistry.
From www.youtube.com
Constant Velocity Calculations YouTube What Is Velocity Constant In Chemistry this equation is a modified form of graham's law. the higher the curve at a given speed, the more molecules travel at that speed. You may, if you wish, read more about the above equation here. For example, many molecules have speeds around 500. a first order (or unimolecular) reaction then is one in which the velocity. What Is Velocity Constant In Chemistry.
From www.slideserve.com
PPT Introduction to Applied Physics PowerPoint Presentation, free download ID1755907 What Is Velocity Constant In Chemistry this is the equation you need to use: a first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one. a velocity is constant when both its magnitude and direction do not change over time. Thus quadrupling the temperature of a given gas doubles the rms. What Is Velocity Constant In Chemistry.
From www.wikihow.com
How to Calculate Average Velocity 12 Steps (with Pictures) What Is Velocity Constant In Chemistry Thus quadrupling the temperature of a given gas doubles the rms velocity of the molecules. a first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one. In other words, this is when the rate of change of position of an. explain the relationships between instantaneous velocity,. What Is Velocity Constant In Chemistry.
From www.scribd.com
Chem Lab A Velocity Constant Titration PDF Chemical Reactions Reaction Rate What Is Velocity Constant In Chemistry V = (3rt) / m. this is the equation you need to use: if the volume is held constant, the increased speed of the gas molecules results in more frequent and more forceful collisions with. In other words, this is when the rate of change of position of an. the rms velocity is directly proportional to the. What Is Velocity Constant In Chemistry.
From chemistnotes.com
Average Velocity, Root Mean Square Velocity, and Most Probable Velocity Chemistry Notes What Is Velocity Constant In Chemistry a velocity is constant when both its magnitude and direction do not change over time. explain the relationships between instantaneous velocity, average velocity, instantaneous speed,. Thus quadrupling the temperature of a given gas doubles the rms velocity of the molecules. this is the equation you need to use: In other words, this is when the rate of. What Is Velocity Constant In Chemistry.
From brainly.in
what is molecularity and order of reaction? write unit of velocity constant for zero order What Is Velocity Constant In Chemistry In other words, this is when the rate of change of position of an. For example, many molecules have speeds around 500. You may, if you wish, read more about the above equation here. this equation is a modified form of graham's law. a velocity is constant when both its magnitude and direction do not change over time.. What Is Velocity Constant In Chemistry.
From ar.inspiredpencil.com
Max Planck Constant What Is Velocity Constant In Chemistry a velocity is constant when both its magnitude and direction do not change over time. V = (3rt) / m. this is the equation you need to use: In other words, this is when the rate of change of position of an. if the volume is held constant, the increased speed of the gas molecules results in. What Is Velocity Constant In Chemistry.
From classnotes.org.in
MaxwellBoltzmann Distribution Chemistry, Class 11, States of Matter What Is Velocity Constant In Chemistry the rms velocity is directly proportional to the square root of temperature and inversely proportional to the square root of molar mass. In other words, this is when the rate of change of position of an. this is the equation you need to use: the higher the curve at a given speed, the more molecules travel at. What Is Velocity Constant In Chemistry.
From www.slideserve.com
PPT Physics 218 PowerPoint Presentation, free download ID6287469 What Is Velocity Constant In Chemistry V = (3rt) / m. You may, if you wish, read more about the above equation here. In other words, this is when the rate of change of position of an. this equation is a modified form of graham's law. Thus quadrupling the temperature of a given gas doubles the rms velocity of the molecules. the higher the. What Is Velocity Constant In Chemistry.
From www.youtube.com
Velocity Formula Science 0075 Lethbridge College YouTube What Is Velocity Constant In Chemistry this is the equation you need to use: You may, if you wish, read more about the above equation here. this equation is a modified form of graham's law. the rms velocity is directly proportional to the square root of temperature and inversely proportional to the square root of molar mass. a velocity is constant when. What Is Velocity Constant In Chemistry.
From empoweryourknowledgeandhappytrivia.wordpress.com
Velocity Formula KnowItAll What Is Velocity Constant In Chemistry The basic idea is that, if you consider each gas. For example, many molecules have speeds around 500. this equation is a modified form of graham's law. It suggests that the velocity (or rate) at which gas molecules move is inversely proportional to the square root of their. Thus quadrupling the temperature of a given gas doubles the rms. What Is Velocity Constant In Chemistry.
From askfilo.com
The unit of the velocity constant of first order reaction is Filo What Is Velocity Constant In Chemistry For example, many molecules have speeds around 500. if the volume is held constant, the increased speed of the gas molecules results in more frequent and more forceful collisions with. The basic idea is that, if you consider each gas. explain the relationships between instantaneous velocity, average velocity, instantaneous speed,. V = (3rt) / m. the rms. What Is Velocity Constant In Chemistry.
From www.showme.com
Frequency, Wavelength, and Speed of Light Constant Science, Chemistry, Waves ShowMe What Is Velocity Constant In Chemistry the higher the curve at a given speed, the more molecules travel at that speed. Thus quadrupling the temperature of a given gas doubles the rms velocity of the molecules. a first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one. It suggests that the velocity. What Is Velocity Constant In Chemistry.
From www.slideserve.com
PPT Physical Chemistry PowerPoint Presentation, free download ID808396 What Is Velocity Constant In Chemistry this equation is a modified form of graham's law. The basic idea is that, if you consider each gas. You may, if you wish, read more about the above equation here. the rms velocity is directly proportional to the square root of temperature and inversely proportional to the square root of molar mass. For example, many molecules have. What Is Velocity Constant In Chemistry.
From www.youtube.com
CHEMISTRY 101 Root mean square velocity of gas molecules YouTube What Is Velocity Constant In Chemistry a velocity is constant when both its magnitude and direction do not change over time. this equation is a modified form of graham's law. The basic idea is that, if you consider each gas. a first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one.. What Is Velocity Constant In Chemistry.
From exocelufe.blob.core.windows.net
What Is The Difference Between Constant Velocity And Constant Speed at Jeff Gonzales blog What Is Velocity Constant In Chemistry the higher the curve at a given speed, the more molecules travel at that speed. V = (3rt) / m. It suggests that the velocity (or rate) at which gas molecules move is inversely proportional to the square root of their. You may, if you wish, read more about the above equation here. this equation is a modified. What Is Velocity Constant In Chemistry.
From www.youtube.com
Constant Velocity Graph YouTube What Is Velocity Constant In Chemistry the rms velocity is directly proportional to the square root of temperature and inversely proportional to the square root of molar mass. You may, if you wish, read more about the above equation here. a velocity is constant when both its magnitude and direction do not change over time. explain the relationships between instantaneous velocity, average velocity,. What Is Velocity Constant In Chemistry.
From www.youtube.com
Chemistry Units of velocity constant YouTube What Is Velocity Constant In Chemistry It suggests that the velocity (or rate) at which gas molecules move is inversely proportional to the square root of their. In other words, this is when the rate of change of position of an. You may, if you wish, read more about the above equation here. V = (3rt) / m. a first order (or unimolecular) reaction then. What Is Velocity Constant In Chemistry.
From www.theengineeringprojects.com
What is Velocity? Definition, SI Unit, Examples & Applications The Engineering Projects What Is Velocity Constant In Chemistry the rms velocity is directly proportional to the square root of temperature and inversely proportional to the square root of molar mass. The basic idea is that, if you consider each gas. a velocity is constant when both its magnitude and direction do not change over time. It suggests that the velocity (or rate) at which gas molecules. What Is Velocity Constant In Chemistry.
From www.youtube.com
Constant Velocity with graphical explanation YouTube What Is Velocity Constant In Chemistry It suggests that the velocity (or rate) at which gas molecules move is inversely proportional to the square root of their. Thus quadrupling the temperature of a given gas doubles the rms velocity of the molecules. In other words, this is when the rate of change of position of an. the higher the curve at a given speed, the. What Is Velocity Constant In Chemistry.
From www.tessshebaylo.com
Equation For Velocity Chemistry Tessshebaylo What Is Velocity Constant In Chemistry this equation is a modified form of graham's law. V = (3rt) / m. the rms velocity is directly proportional to the square root of temperature and inversely proportional to the square root of molar mass. a first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration. What Is Velocity Constant In Chemistry.
From www.slideserve.com
PPT Motion with Constant Velocity in 1D PowerPoint Presentation, free download ID2615973 What Is Velocity Constant In Chemistry It suggests that the velocity (or rate) at which gas molecules move is inversely proportional to the square root of their. explain the relationships between instantaneous velocity, average velocity, instantaneous speed,. this equation is a modified form of graham's law. V = (3rt) / m. a first order (or unimolecular) reaction then is one in which the. What Is Velocity Constant In Chemistry.
From www.youtube.com
Velocity and Acceleration Constant Velocity YouTube What Is Velocity Constant In Chemistry explain the relationships between instantaneous velocity, average velocity, instantaneous speed,. a first order (or unimolecular) reaction then is one in which the velocity of the reaction is proportional to the concentration of one. a velocity is constant when both its magnitude and direction do not change over time. In other words, this is when the rate of. What Is Velocity Constant In Chemistry.
From lambdageeks.com
What Is Constant Negative Velocity Why, How, When and Problem Examples Lambda Geeks What Is Velocity Constant In Chemistry if the volume is held constant, the increased speed of the gas molecules results in more frequent and more forceful collisions with. explain the relationships between instantaneous velocity, average velocity, instantaneous speed,. In other words, this is when the rate of change of position of an. It suggests that the velocity (or rate) at which gas molecules move. What Is Velocity Constant In Chemistry.